At the center of the licensing deal is an NLRP3 inhibitor that has shown “encouraging efficacy in acute inflammation models,” according to TransThera, indicating its potential in various metabolic and inflammatory diseases.
Manifold will use its tissue-targeting shuttle technology to help Roche develop new therapeutics for diseases of the central nervous system.
A month after sparking optimism for patients with Huntington’s disease with highly positive data for AMT-130, uniQure revealed Monday that the FDA may be changing its tune on the evidence required for an approval application, a change of direction Stifel called “very challenging” for uniQure.
Pfizer has filed two separate lawsuits in an effort to stop Novo Nordisk’s unsolicited bid to acquire obesity biotech Metsera.
Vinay Prasad, chief of the FDA’s Center for Biologics Evaluation and Research, is planning to publish a paper this month to outline his office’s thinking on accelerating gene editing reviews.
Novo Nordisk, under new CEO Maziar Mike Doustdar, has a new attitude. It’s making Pfizer livid.
FEATURED STORIES
The industry sector focused on aging is only about 10 years old, but acting on what scientists already know, a new crop of biotechs, backed by investors, are taking a disease-centric approach to extending the human lifespan.
Analysts said the deal with Novo was likely giving Hims “‘credibility’ or increased consumer traffic,” adding that the “litigation risk is back on the table” now that the Danish pharma has stepped away.
TIGIT-targeting therapies have largely disappointed in recent months, with failed studies, terminated partnerships and shuttered businesses. Here are five biopharma players staying alive with differentiated candidates against the once promising immuno-oncology target.
Slashing adverse drug reactions through pharmacogenetics and advanced AI could help rehabilitate the pharmaceutical industry’s reputation amid mounting criticism.
Analysts believe that Gilead’s new PrEP drug Yeztugo could reach peak sales of $4.5 billion. Not if GSK has anything to say about it.
Why did two private equity firms with more than $460 billion under management want a little old gene therapy biotech called bluebird bio? We wanted to know.
FROM BIOSPACE INSIGHTS
BioSpace’s 2022 Salary Report explores the average salaries and salary trends of life sciences professionals. Though movement in the labor market slowed during the pandemic, recovery has been swift and employers are once again having to cope with a highly competitive talent market.
LATEST PODCASTS
In this episode of Denatured, BioSpace’s Head of Insights Lori Ellis, Miguel Forte and Ali Pashazadeh speculate on the impending Trump administration, discuss current challenges faced by CEOs and weigh investment in GLP-1s.
The Novo-Catalent deal now moving ahead highlights unprecedented investment in manufacturing, while also standing out as an exception to the unspoken rule of keeping M&As to less than $5 billion this year.
GSK, Gilead and Arcellx, Vertex and more present new data at the American Society of Hematology annual meeting just as sickle cell therapies Casgevy and Lyfgenia have a new outcomes-based payment model; Eli Lilly and Novo Nordisk pump new funds into manufacturing; and AbbVie makes a Cerevel comeback while uniQure clears a path toward accelerated approval in Huntington’s disease.
Job Trends
Securing top talent in research and development and manufacturing will remain challenging for life sciences companies in 2024, according to a new report by CBRE.
Subscribe to GenePool
Subscribe to BioSpace’s flagship publication including top headlines, special editions and life sciences’ most important breaking news
SPECIAL EDITIONS
In this deep dive, BioSpace investigates China’s rise as a biotech powerhouse.
In this deep dive, BioSpace explores the next big thing in obesity.
BioSpace did a deep dive into biopharma female executives who navigated difficult markets to lead their companies to high-value exits.
DEALS
-
With an eye toward advancing a novel antibody-drug conjugate for gastrointestinal cancers, ArriVent is the latest biopharma player to ink a deal with a Chinese biotech.
-
Five years ago, Gilead signed a massive deal with Galapagos. After a restructuring, the pharma is still hunting for the potential it saw at the original signing.
-
Biopharma executives shared their thoughts on the potential impacts of the new administration; Annalee Armstrong recaps JPM and her talks with Biogen, Gilead, Novavax and more; Wegovy’s higher dose induces more weight loss; AstraZeneca and Daiichi Sankyo’s Dato-DXd scores its first FDA approval.
-
Biopharma executives make their predictions for the year ahead, from a bold forecast for the return of the megadeal to a plea for the slow, healthy recovery of the industry at large.
-
While investors and analysts push for a deal, Biogen CEO Chris Viehbacher and Head of Development Priya Singhal refuse to make one out of desperation.
WEIGHT LOSS
-
Under Friday’s final ruling anti-obesity medications for weight-loss will remain ineligible for Medicare coverage.
-
Eli Lilly says Indianapolis-based Premier Weight Loss is cracking open auto-injector pens containing its blockbuster drug and repackaging them into separate doses.
-
According to BMO Capital Markets, Rybelsus’ outcomes in SOUL were “inconsistent,” failing to significantly lower cardiovascular death and nonfatal stroke.
-
Compounded versions could make up as much as 40% of the semaglutide market, said Novo Nordisk CEO Lars Fruergaard Jorgensen on Thursday, but the company hopes to win patients over.
-
Lexicon’s LX9851 targets ACSL5, a liver enzyme involved in fat metabolism that helps moderate fat accumulation and slow down gastric emptying.
POLICY
-
The new version of the bill will still need to go through the entire House and Senate.
-
Findings that U.S. companies can sue foreign rivals despite limited business operations in the country could dissuade drug developers from targeting the U.S. market, potentially benefiting domestic producers of biosimilars.
-
The program will bring together experts from across the FDA for a team-based review, rather than having an application move across numerous offices within the agency before getting a yay or nay.
-
District Judge William Young, a nominee of Republican President Ronald Reagan, blasted the Trump administration’s NIH cuts as discriminatory and “bearing down on people of color because of their color.”
-
HHS Secretary Robert F. Kennedy Jr.’s actions in recent months have raised concerns that he is taking a heavy-handed and unilateral approach to vaccine policy in the U.S.
To help you in your job search, here are just a few of the remote job options in the life science industry, along with the qualifications and skills necessary to be successful in each role.
When vetting the qualities of potential candidates at a career fair, listen to and take interest in the unique lived experiences of each job seeker you meet, as well as their skills and qualifications.
Job descriptions are the candidate’s first impression of a company. And if that introduction includes exclusionary language, they’re less likely to apply even if they are the perfect fit for the job.
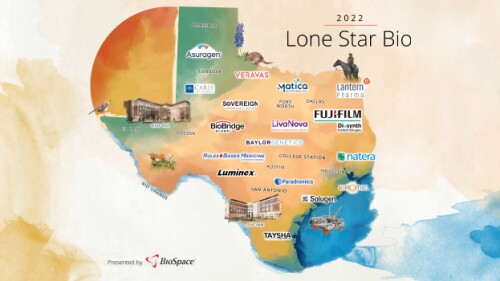
Known as the Lone Star Bio Hotbed, Texas is home to multiple major players in the biopharma and biotech space and is currently a fast-growing hub for the life science industry.
You know it’s important to nail the interview with a potential employer, but it’s what you do after an interview that might really influence your chances of getting the job. Find out how in our guide.
Being laid off from a job is stressful. Fortunately, there are steps you can take to curb some of that stress, relieve financial worries and make finding your next position as smooth a process as possible.
HOTBEDS
REPORTS
In this Employment Outlook report, BioSpace explores current workforce sentiment, job activity trends and the prospective job and hiring outlook for 2025, particularly as it compares to the previous year.
BioSpace’s third report on diversity, equity, inclusion and belonging in life sciences examines dramatic shifts in attitude around diversity initiatives.
CANCER
-
As Q1 2025 earnings season continues, tariffs remain top of mind for pharma CEOs and investors. Meanwhile, the American Association for Cancer Research’s annual event kicks off this year’s oncology conference season. Plus, will the FDA become politicized under HHS Secretary RFK Jr.?
-
Pfizer’s sasanlimab, when used with standard of care, reduced the likelihood of disease recurrence or progression, death due to any cause or persistence of cancer cells by 32% in patients with high-risk non-muscle invasive bladder cancer.
-
The targeted drug release device TAR-200 shows promising response and disease-free survival rates in specific populations of patients with non-muscle-invasive bladder cancer.
-
The FDA is currently reviewing Merck’s sBLA for Keytruda in head and neck cancer, with a target action date of June 23.
-
The transaction is expected to close in the second half of 2025. With the deal, Merck KGaA is adding to its rare disease and oncology pipelines.
NEUROSCIENCE
-
Analysts at BMO Capital Markets said Centessa’s orexin receptor agonist has “best-in-class” potential for narcolepsy, putting the company in a strong position in the $15 billion market.
-
Sangamo, which has been having cash problems, will receive $18 million upfront in licensing fees for its AAV capsid that in preclinical studies has shown the ability to cross the blood-brain barrier.
-
After some high-profile crashes, the one-time biotech darling is inching toward success with its Hunter syndrome treatment, which today began a rolling BLA for accelerated approval.
-
Biopharma leaders react to the forced resignation of CBER Head Peter Marks as RFK Jr.’s promised job cuts begin at the FDA; Novo Nordisk presents mixed results from oral semaglutide in cardiovascular disease; the EU’s Committee for Medicinal Products for Human Use declines to recommend Eli Lilly’s Alzheimer’s drug; and pharma R&D returns grew in 2024.
-
The European Union’s CHMP said that the benefits of the drug, already approved in the U.S., do not outweigh the risk of potentially fatal brain swelling and bleeding.
CELL AND GENE THERAPY
-
On the agenda for the FDA this month are two RNA-based treatments for rare diseases.
-
The search for a partner for zerlasiran is ongoing, according to Silence. In the meantime, the biotech will focus its resources on divesiran, which it is testing for polycythemia vera and other hematologic indications.
-
ITF, IntraBio and Orchard are among the companies that have won FDA nods in the past year for Duchenne muscular dystrophy, Niemann-Pick disease type C, metachromatic leukodystrophy and more.
-
The companies were two years into a four-year, $400 million agreement aimed at developing and marketing gene therapies together.
-
As FDA seeks to rehire some fired employees, Donald Trump threatens to enact tariffs on pharma companies unless they reshore manufacturing; another lawsuit hits the complex GLP-1 compounding space as Eli Lilly offers expanded Zepbound options; and struggling gene therapy biotech bluebird bio goes private in an attempt to stay solvent.