Now that global responses are fully mobilized, what is being done to identify and develop drugs and vaccines against this novel coronavirus?
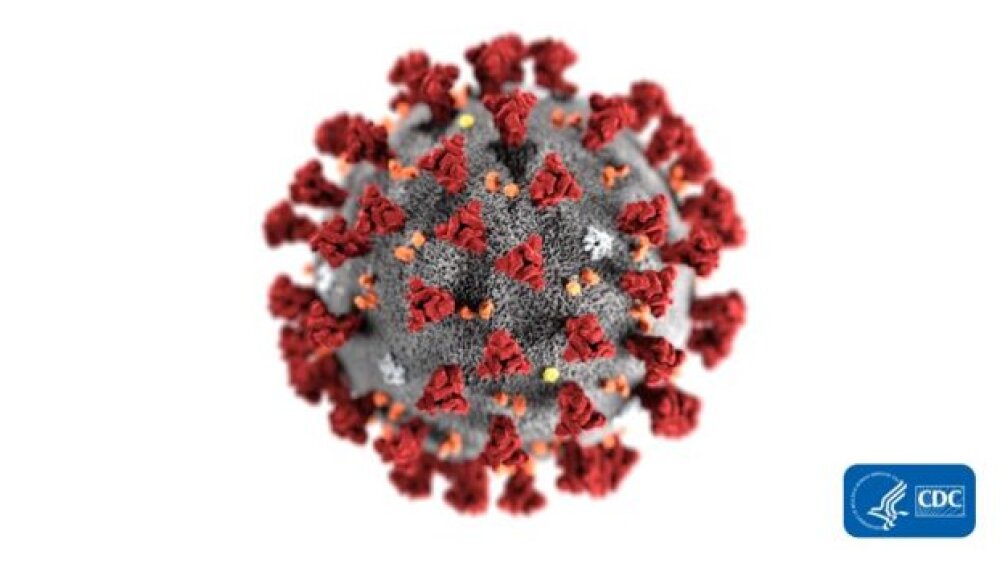
“This illustration, created at the Centers for Disease Control and Prevention (CDC), reveals ultrastructural morphology exhibited by coronaviruses. Note the spikes that adorn the outer surface of the virus, which impart the look of a corona surrounding the virion, when viewed electron microscopically.” Credit: CDC Public Health Image Library (PHIL) #23312.
The novel coronavirus (2019-nCoV), which originated in Wuhan, China in December 2019, has been making headlines left and right as the ticker climbs of those who have been sickened and died from the virus. On February 11, the World Health Organization (WHO) officially named the disease caused by the 2019-nCoV virus COVID-19, which stands for coronavirus disease 2019.
As of February 11, there were 44,836 confirmed COVID-19 cases worldwide, 4,686 people recovered, and 1,113 deaths (2.5 percent mortality) worldwide, according to the live COVID-19 global tracker created by Johns Hopkins Center for Systems Science and Engineering (CSSE) that combines data from the WHO, US Centers for Disease Control and Prevention (CDC), European CDC (ECDC), and China’s National Health Commission (NHC). The vast majority of these cases have been in mainland China (44,360 cases, 4,636, recovered, and 1,111 deaths), especially in the Hubei province (whose capital is Wuhan). According to Chinese health officials at a WHO meeting, 80 percent of those who died in China were over 60 years old and 75 percent had an underlying condition.
While all the media buzz may seem alarming and the virus is causing serious illness (and even death), the risk to the American public is very low. The majority of cases in the US were contracted while traveling in China and robust detection and quarantine efforts in the US greatly reduce the risk of community-spread infection.
The virus’ risks should be taken seriously but put into context with other upper respiratory illnesses, such as the flu, to not cause panic. The CDC recommends “no additional precautions [for the general public] at this time beyond the simple daily precautions that everyone should always take,” such as washing your hands regularly, cleaning and disinfecting objects regularly, getting your flu shot, avoiding contact with sick people, and staying home if you are sick.
For more information on the current outbreak, check out the live updates from the CDC, WHO, European CDC, Nature, WebMD, and Newsweek.
The international response
The swift, intense, and collaborative international response is like a cool breeze of fresh air – it stands in stark contrast with the slower response to the 2002 severe acute respiratory syndrome (SARS) outbreak, a severe disease caused by another coronavirus. Advances in genetic sequencing technology, public health communication, and viral outbreak preparedness are shining through this current coronavirus outbreak.
“Improved technology, pressure tested simulated pandemic situations, and stronger relationships that encourage collaboration between organizations and governments during emergency situations have been a bright spot so far in this novel coronavirus outbreak,” Carl Hansen, PhD, Director and CEO of AbCellera, a Vancouver-based biotech company who is developing antibody drugs against 2019-nCoV, told BioSpace. “The global effort against this virus isn’t competitive so far and is moving forward for all the right reasons.”
On January 30, 2020, the WHO declared the 2019-nCoV outbreak a Public Health Emergency of International Concern (PHEIC). The US followed suit on January 31 by declaring the outbreak a Public Health Emergency (PHE). Both declarations call for action and release resources needed to handle a public health crisis.
Now that global responses are fully mobilized, what is being done to identify and develop drugs and vaccines against this novel coronavirus? First, let us go back to the basics and a brief history of coronaviruses.
What are coronaviruses?
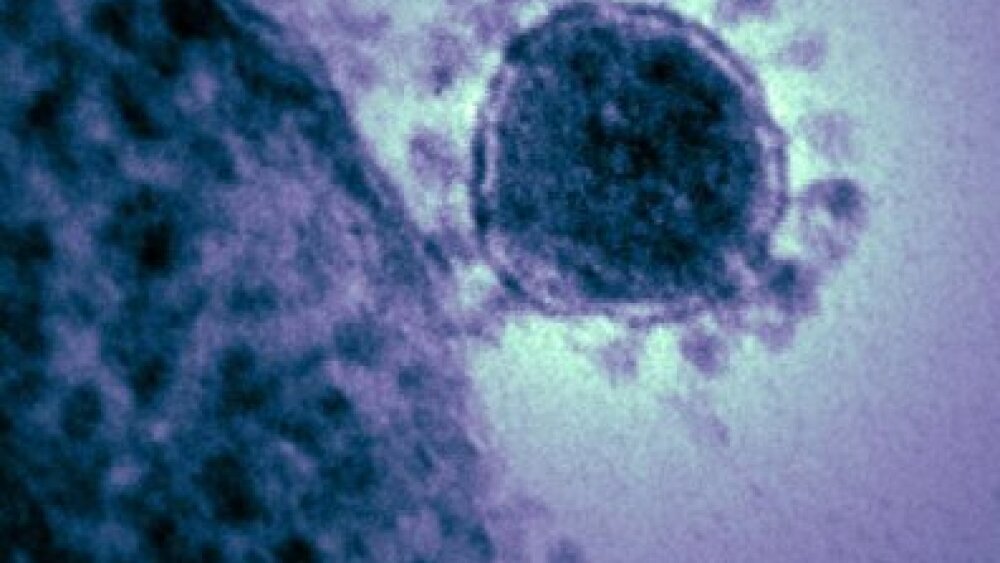
Coronaviruses are a family of viruses that can infect humans and other animals. “Corona” is Latin for “crown” – these viruses were named for the crown-like structure of their outer proteins (seen in the picture of a MERS virus particle below).
“Produced by the National Institute of Allergy and Infectious Diseases (NIAID), this highly magnified, digitally colorized transmission electron microscopic (TEM) image, reveals ultrastructural details exhibited by a single, spherical-shaped, Middle East respiratory syndrome coronavirus (MERS-CoV) virion.” Credit: CDC Public Health Image Library (PHIL) #18114.
While coronaviruses are the infamous culprits behind the 2002 SARS and 2012 Middle East respiratory syndrome (MERS) diseases, they also come in less severe varieties. In fact, you have probably been sickened by one of the handful of less virulent strains of coronavirus, causing cold symptoms like stuffy nose, cough, and sore throat.
Four types of coronaviruses are endemic globally and cause 10-30 percent of upper respiratory infections in adults. While there is no way to tell which virus is causing your cold symptoms, typical coronavirus infections are more common in the fall and winter in the US (typical cold and flu season) and are more common in young children.
SARS and MERS – how are they related to 2019-nCoV?
Rarely, a coronavirus that normally infects an animal mutates and infects humans; this creates a new human coronavirus that tends to cause more severe symptoms. This was the case for the coronavirus strains that cause SARS, MERS, and now the novel coronavirus outbreak. While the initial 2019-nCoV cases were linked to the Huanan Seafood Wholesale Market (which sold live fish, animals, and birds), the market has since been closed down before the animal source of the new coronavirus could be determined.
SARS was first seen in China in November 2002, causing a “worldwide outbreak in 2002-2003 with 8,098 probable cases including 774 deaths” (9.6 percent mortality). There have been no cases of SARS since 2004.
MERS was first seen in Saudi Arabia in 2012, causing an outbreak in countries in or near the Arabian Peninsula. There are still new reports of MERS currently, with the WHO reporting two new laboratory-confirmed MERS cases in the United Arab Emirates from January 9 and 13. From 2012 to January 15, 2020, there have been 2,506 MERS cases and 862 MERS-associated deaths (34.4 percent mortality), according to the WHO. The CDC estimates that about 3-4 out of every 10 patients who have MERS have died.
Is there a 2019-nCoV-specific diagnostic test available?
Yes! Because the genetic sequence of this novel coronavirus was rapidly identified and widely shared online (researchers from Fudan University in Shanghai posted the sequence on Virological.org on January 10), researchers were able to quickly analyze multiple patient’s samples and begin developing a diagnostic test.
On January 17, the WHO published interim guidance for a polymerase chain reaction (PCR)-based diagnostic lab test. Respiratory samples (nose or mouth swabs, sputum, or bronchoalveolar lavage) are collected from suspected COVID-19 patients and all the DNA in the sample is amplified using PCR so it can be analyzed. If a DNA sequence that matches the 2019-nCoV is found, then the patient can be diagnosed with the novel virus.
On February 4, the US Food and Drug Administration (FDA) issued an emergency use authorization (EUA) that allowed the CDC’s PCR-based laboratory test (the 2019-nCoV Real-Time RT-PCR Diagnostic Panel) to be used across the US in any CDC-qualified lab. Previously, the test could only be performed at CDC laboratories.
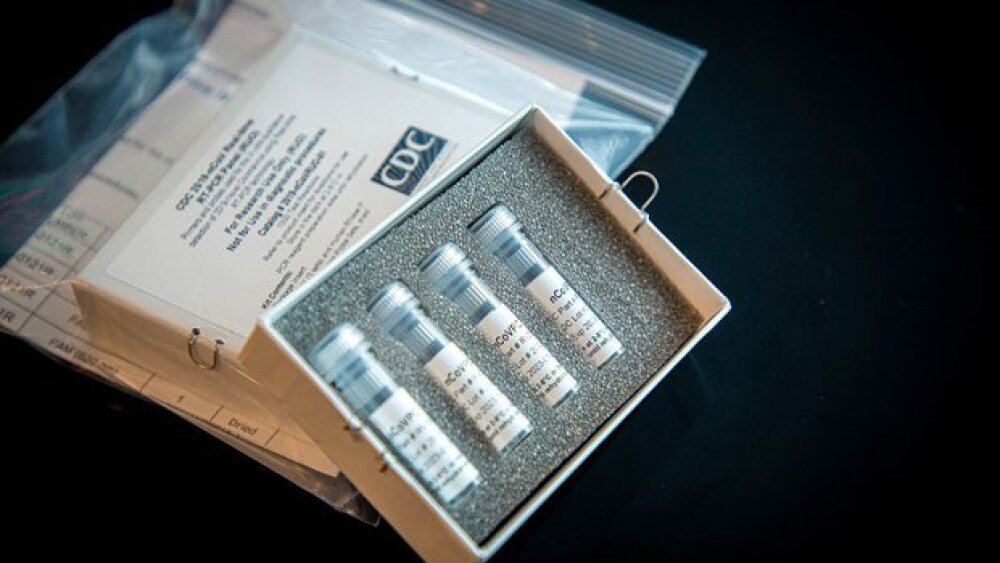
On February 5, CDC RT-PCR diagnostic panel kits (shown below) were made available to order by both domestic and international partners.
A picture of the CDC’s RT-PCR diagnostic panel kit available for order. These kits can be shipped to US CDC-qualified labs, Department of Defense (DOD) labs, and certain international labs. Credit: cdc.gov
AbCellera’s approach to developing antibody drugs against 2019-nCoV
On January 27, the FDA published a press release promoting “collaboration with interagency partners, product developers, international partners and global regulators to expedite development and availability of medical products needed to diagnose, treat, mitigate and prevent” this novel coronavirus outbreak.
One such company working with the US government to rapidly develop drugs against the novel coronavirus is AbCellera. Their single-cell microfluidics platform can be used to identify therapeutic antibodies against a broad range of viruses.
In fact, they have been prepping for just this situation – AbCellera partnered with the Defense Advanced Research Projects Agency (DARPA) in March 2018 to develop “rapid countermeasures” against viral pandemic outbreaks. Under their Pandemic Prevention Platform (P3), DARPA will support AbCellera’s development and testing of their microfluidics platform to establish it as an end-to-end platform ready to be put into action when a viral pandemic strikes. The P3 program aims to develop a platform that could stop a pandemic within 60 days – a highly ambitious goal given the typically large amount of time needed to identify, isolate, synthesize, purify, manufacture, and test any new drug, especially a biologic drug like an antibody.
“AbCellera’s platform allows for a deep and rapid search of natural immune responses to rapidly identify antibodies against any virus from patient samples,” explained Hansen. “It is an integrated end-to-end solution from antibody generation and discovery all the way through protein engineering for drug discovery.”
Blood samples from patients who have been infected with the virus of interest and recovered are screened to identify antibodies that can recognize and bind the virus. B-cells, immune cells that make antibodies, are analyzed individually in microfluidic chambers with one cell per nanoliter volume (a minuscule scale compared to typical benchtop assays). As each B-cell produces its unique antibody, they can all be separately tested for their ability to bind to the viral proteins, usually envelope proteins on the outside of the virus.
This microfluidics approach allows them to screen millions of antibodies in a high-throughput manner in a matter of hours because each chamber requires nanoscale amounts of liquids (effectively generating high concentrations of antibodies in a short time) and multiple chambers can be run simultaneously.
Once the antibodies that best bind the viral proteins are identified, the B-cells that specifically secrete those antibodies are selected and broken open. This releases the RNA molecules that encode for the antibodies, which are then sequenced to obtain each antibodies’ unique genetic code. This process can generate hundreds to thousands of unique antibody sequences per run.
The antibodies’ RNA is then cloned and expressed to generate large amounts of antibodies for biochemical analyses. The best antibody drug candidates are then shipped to AbCellera’s collaborators at the National Institutes of Health (NIH) where the antibodies can be tested in further cellular assays and in animal models.
This whole process happens at unprecedented speeds – a mere 12 days from screening a blood sample to shipping potential antibody drugs to collaborators for further testing, and about two months from blood sample to animal-tested antibody drug candidates ready for human testing.
“Generating antibody sequences from blood samples can happen as quickly as four days when the company is operating at maximum speed,” Hansen said. “Cloning, expression, and biochemical analysis of the selected antibodies can then happen as quickly as eight days after, for a total time of less than two weeks.”
Manufacturing the antibody drugs will be the next hurdle in terms of the time required (usually at least six months) and specialized resources needed.
AbCellera has already pressure tested their platform by simulating a MERS outbreak response and a pandemic influenza response. Using samples from MERS-infected alpacas (a relative of the camel, the natural animal reservoir for MERS-CoV), the company was able to identify thousands of MERS-CoV-binding antibodies in one afternoon, then obtain 355 unique antibody sequences in 3.8 days. During its “test run” against a simulated pandemic influenza outbreak, the company was able to go from influenza antibody discovery from human samples to administering antibody therapeutics that proved effective in rodent models in just 55 days.
Now, AbCellera is getting to put their platform to the test in the real world with the novel coronavirus. Currently, they are waiting to receive samples from patients who have recovered from 2019-nCoV (which should arrive to them within 1-3 weeks) and preparing their platform to run at maximum speed. Other groups and companies are currently shuffling around resources in preparation for antibody manufacturing down the line.
“We are excited and honored to be a part of this work,” commented Hansen. “Our team has put in the hard work to create a universal rapid response platform and has invested time and effort into building the networks needed in emergency situations. We would like to thank everyone who has contributed to putting us in an ideal position to perform this very important research.”
What else is being done to develop drugs against 2019-nCoV?
Other companies are jumping into the race towards an effective coronavirus drug. Regeneron partnered with the US Department of Health and Human Services (HHS) to also identify and develop new antibody drugs against 2019-nCoV. Regeneron previously leveraged its technology and resources in a similar partnership during recent Ebola outbreaks to generate an experimental drug combination against the Ebola virus.
Vir Biotechnology, a San Francisco-based startup, is rapidly testing their “library of fully-human monoclonal antibodies” against SARS and MERS to see if they have activity against 2019-nCoV.
Gilead is also working with global health agencies to examine if its investigational antiviral drug remdesivir is useful against 2019-nCoV. Remdesivir was originally developed as a treatment for Ebola but has since shown antiviral activity against other viruses, including coronaviruses like MERS-CoV. In fact, remdesivir and chloroquine (a common anti-malarial drug that has broad-spectrum antiviral activity) were shown to effectively inhibit 2019-nCoV replication in the lab.
Phase 3 clinical trials testing remdesivir in 308 adults with mild to moderate 2019-nCoV illness and 452 adults with severe 2019-nCoV illness have recently begun at Jinyintan Hospital in Wuhan.
Another possible way to combat this novel coronavirus is to repurpose existing antiviral drugs. After receiving large doses of the flu drug oseltamivir (Tamiflu) and two HIV drugs lopinavir and ritonavir (Kaletra, also known as Aluvia), a 70-year-old patient with severe 2019-nCoV illnesses in Thailand improved.
“This is not the cure, but the patient’s condition has vastly improved,” Dr. Kriangska Atipornwanich, a lung specialist at Rajavithi Hosptial in Bangkok, told Reuters. “. From testing positive for 10 days under our care, after applying this combination of medicine the test result became negative within 48 hours.”
Chinese health authorities are recommending Kaletra and nebulized alpha-interferon (a drug that inhibits viral replication) to treat 2019-nCoV-induced pneumonia. AbbVie, the manufacturer of Kaletra, pledged to donate about $1.5 million worth of Kaletra to patients.
Kaletra showed promise previously in SARS patients by significantly lowering the adverse clinical outcomes or death in treated patients compared to historical controls. Kaletra, in combination with interferon beta-1b (a certain type of immune cell called a cytokine that boosts the immune system’s response), is also currently in a Phase 2/3 trial in an estimated 194 MERS patients.
A randomized control trial was quickly initiated in China (beginning on January 18) to study the safety and efficacy of Kaletra in patients hospitalized with 2019-nCoV infections. Two other Phase 4 trials in China studying antivirals in 2019-nCoV infected patients are listed on ClinicalTrials.gov: one studying Kaletra and the flu drug umifenovir (Arbidol) in an estimated 125 adults with 2019-nCoV illness, and another studying Kaletra, Tamiflu, or abidol hydrochloride in an estimated 400 adults with 2019-nCoV-induced pneumonia.
What about developing a vaccine against 2019-nCoV?
“Although vaccines are the most cost-effective for large-scale protection, they take a longer time to develop,” Hansen commented. “Vaccines are complementary to the quicker and more emergency response-related antibody drug approach.”
Although the vaccine development process is getting speedier (taking about 20 months from genomic sequence identification to Phase 1 clinical trials for a SARS-CoV DNA vaccine compared to just 3.25 months for other viral diseases more recently), it will likely take months before the vaccine is ready for testing in humans.
The National Institute of Allergy and Infectious Diseases (NIAID) is collaborating with Moderna to develop an mRNA vaccine. Moderna isn’t wasting any time – they have already designed and manufactured the first batch of an experimental 2019-nCoV vaccine in a mere 25 days. They plan to send the initial batch to the NIH for initial clinical testing soon.
The NIAID-Moderna partnership will be funded in part by the Coalition for Epidemic Preparedness Innovations (CEPI), which is also funding two other 2019-nCoV vaccine development programs at the University of Queensland in Australia and Inovio Pharmaceuticals.
Inovio, who was involved in the MERS and Zika outbreak responses, hope to leverage their “DNA medicine platform” to bring a vaccine to human trials within months, similar to their Zika vaccine candidate (which went into trials in just seven months). In fact, they plan to enter human trials with the potential 2019-nCoV vaccine, called INO-4800, by early this summer.
Johnson & Johnson has also thrown their hat into the vaccine development ring. The company estimates that it will take about one year to advance from initiation of vaccine development to human clinical trials.
Despite the seemingly breakneck speed of companies and organizations working to develop drugs and vaccines against this novel coronavirus and how much technology and viral emergency response coordination have improved, it will likely be months before a new drug or vaccine are ready for testing in humans.
Fortunately, the rapid implementation of outbreak control measures, initiation of drug and vaccine development, and launching of off-label clinical trials is bringing us ever closer to finding drugs effective against this novel coronavirus.