Latest News
& Press Releases
Browse the latest news from BioSpace, and press releases from around the industry. Want to filter by date, keyword, and more? Search here.
TOP STORIES
Biohaven has suffered a few setbacks in recent months, including an FDA rejection and a missed $150 million benchmark payment, but CEO Vlad Coric looked for the brighter side at JPM, specifically emphasizing a serendipitous discovery that could get the company in the obesity game.
In November, Pfizer was reportedly looking to divest its stake in BioNTech, though the German biotech at the time denied these rumors.
The arrangement will boost AstraZeneca’s cell therapy portfolio as the pharma targets $80 billion in revenue by 2030.
The star of the acquisition, anti-IgE antibody ozureprubart, is being tested as a prophylactic treatment for food allergies, potentially setting up a competition for GSK with Roche’s Xolair.
The companies have an expansive clinical program for the mRNA neoantigen therapy intismeran autogene in combination with immuno-oncology heavyweight Keytruda.
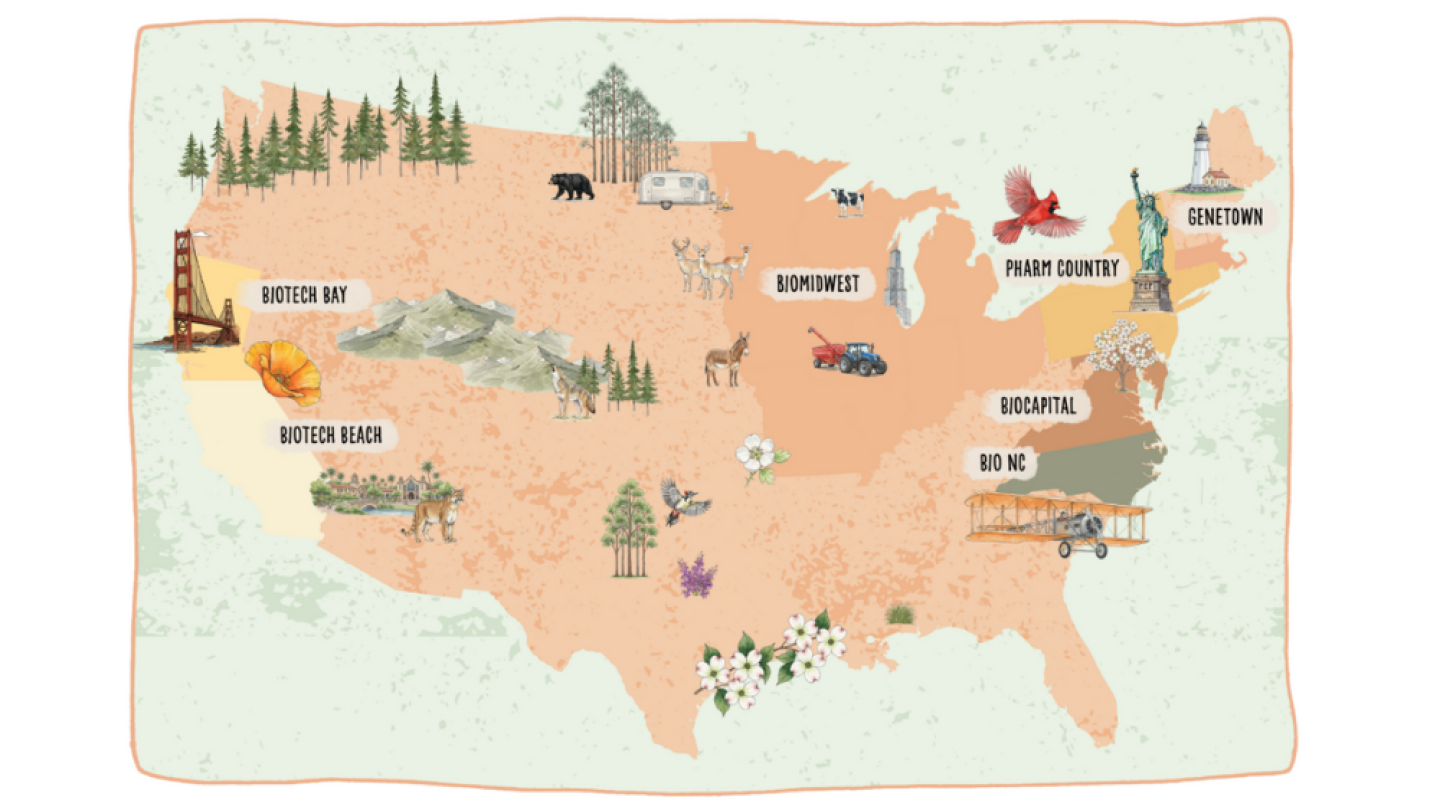
BioSpace has revealed its new 2026 Hotbed Maps, showcasing seven regional life sciences hot spots.
The initiative could tackle the first-mover disadvantage some CDMOs believe deters early customers, but leaders at companies including Novo Nordisk see hurdles to implementing the changes.
Henry Gosebruch, who has $3.5 billion in capital to deploy, is thinking broad as he steers the decades-old biotech out of years of turmoil.
In this bonus episode, BioSpace’s Vice President of Marketing Chantal Dresner and Careers Editor Angela Gabriel take a look at Q4 job market performance and what it signals for 2026.
Follow along as BioSpace tracks job cuts and restructuring initiatives.
Two more biopharma companies—the hair-growth specialist Veradermics and cancer-focused Eikon Therapeutics—have announced their IPOs this year. Meanwhile, Aktis Oncology began trading publicly earlier this month.
Despite the late-stage miss, analysts maintained confidence in the Epkinly program, with Truist Securities saying the result “doesn’t waver our optimism” regarding the bispecific antibody’s ongoing frontline trial.
PRESS RELEASES
Clinical Trial has Advanced to Fifth and Final Cohort at Maximum Dose of INSTASYL PH-762 in Skin Cancer Trial Positive Pathology Results at Maximum Dose: 100% Tumor Clearance (Complete Response) in One Patient, Greater than 90% (Near Complete Response) in Second Patient, Greater than 50% (Partial Response) in Third Patient Safety Monitoring Committee Issues Favorable Review of Safety Data at Maximum Dose of INTASYL PH-762 Warrant Inducement Financing in November 2025 for Expected Net Proceeds Totaling Approximately $12.1 million, Extending Cash Runway into the First Half of 2027
Session led by neurosurgeon Dr. Fraser Henderson and Hemostemix CEO Thomas Smeenk for the University of Florida’s Department of Surgery, Division of Vascular Surgery & Endovascular Therapy (Gainesville)
Once issued, this new U.S. patent covers the use of CardiolRx™ and CRD-38 for a broad range of cardiac disorders, including atherosclerosis and heart failure, significantly expanding intellectual property protection in the world’s largest pharmaceutical market. This allowance fortifies Cardiol’s global intellectual property portfolio, adding to granted and pending patent applications in Europe, Japan, Canada, Australia, and China.
Management to host conference call at 8:00 AM Eastern time on Thursday, November 13, 2025
Near-term applications include combinations with kinase inhibitors (KIs) that achieve enhanced concentration at target sites and synergistic delivery of multiple KIs to the same location