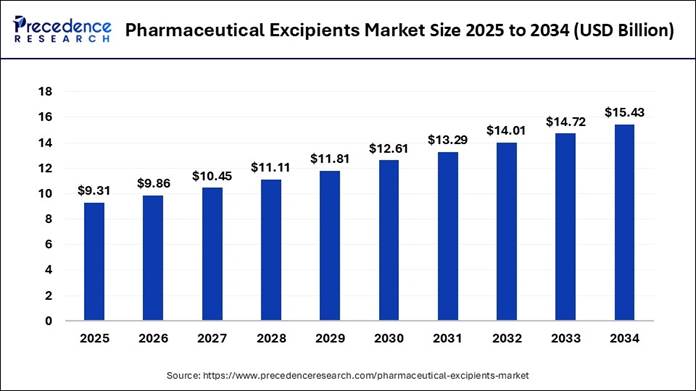
According to Precedence Research, the pharmaceutical excipients market size is calculated at USD 9.31 billion in 2025 and is expected grow from USD 9.86 billion in 2026 to nearly USD 15.43 billion by 2034. In terms of CAGR, the market is expanding at a strong compound annual growth rate (CAGR) of 5.75% from 2025 to 2034.
Pharmaceutical excipients, which are pharmacologically inactive, are crucial in the preparation, stability, and efficacy of drugs. They are added together with active pharmaceutical ingredients (APIs) to facilitate the manufacturing process as well as to ensure that the end product is safe, effective, and user-friendly.
Note: This report is readily available for immediate delivery. We can review it with you in a meeting to ensure data reliability and quality for decision-making.
📥 Download Sample Pages for Informed Decision-Making 👉 https://www.precedenceresearch.com/sample/1761
Market Key Takeaways
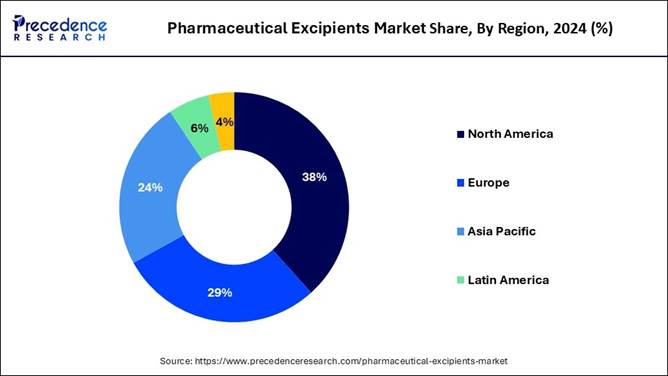
🔹North America accounted for the largest market share of 38.32% in 2024.
🔹 By excipient type, the lactose-based excipients segment contributed the highest market share of 41.26% in 2024.
🔹 By functionality, the fillers and diluents segment generated the largest market share in 2024.
🔹 By functionality, the coating agents segment is expected to grow significantly CAGR over the forecast period.
🔹 By excipient type, the lactose-based excipients segment held a major share in 2024.
🔹 By excipient type, the cellulose-based segment is growing at a notable CAGR from 2025 to 2034.
Pharmaceutical Excipients Market Size, Growth and Forecast
🔹 Market Size in 2025: USD 9.31 Billion
🔹 Market Size in 2026: USD 9.86 Billion
🔹 Market Size by 2034: USD 15.43 Billion
🔹 CAGR (2025-2034): 5.75%
🔹U.S. Market Size in 2025: USD 3.08 Billion
🔹U.S. Market Size by 2034: USD 5.32 Billion
🔹 Largest Region in 2024: North America
🔹 Fastest Growing Region: Europe
Pharmaceutical Excipients Market Regional Landscape
🔹 The North America pharmaceutical excipients market was valued at USD 3.15 billion in 2024 and is projected to reach approximately USD 5.66 billion by 2034, registering a CAGR of 6.04% from 2025 to 2034. The region continues to dominate due to its advanced pharmaceutical manufacturing infrastructure, strong R&D base, and presence of leading global drug makers.
🔹 The Europe pharmaceutical excipients
market stood at USD 2.35 billion in 2024 and is anticipated to expand to around
USD 3.95 billion by 2034, growing at a steady CAGR of 5.30%. Rising investments
in sustainable formulations and the growing biologics sector are driving market
expansion across key economies such as Germany, the U.K., and France.
🔹 The Asia Pacific pharmaceutical
excipients market accounted for USD 1.94 billion in 2024 and is forecast to
grow at the fastest rate, with a CAGR of 6.53% from 2025 to 2034, reaching
about USD 3.65 billion by 2034. The region’s growth is fueled by large-scale
generic drug production, favorable government policies, and increasing
pharmaceutical exports from India and China.
🔹 The Middle East and Africa (MEA) pharmaceutical excipients market was valued at USD 294.40 million in 2024 and is projected to rise to USD 454.85 million by 2034, expanding at a CAGR of 4.44%. Market growth is supported by healthcare infrastructure development, expanding drug manufacturing capacity, and gradual adoption of international quality standards.
Pharmaceutical Excipients Market Overview and Industry Potential
Innovation in Drug Formulation Propels Excipients Market Forward
The pharmaceutical excipients market is anticipated to experience fast-paced growth in the coming years, akin to the increased need for stable, effective, and easier-to-consume medicine in recent years. Also, the innovations in biologics and increasing patient base across the world are actively contributing to the industry's growth. Moreover, the manufacturers are heavily investing in the development of multifunctional and plant-based excipients in the current period.
Eco-Friendly, Bio-Derived Excipients Set to Transform Drug Delivery
Developing functional and sustainable excipients that go beyond basic roles is expected to create lucrative opportunities for the manufacturer in the upcoming years. Manufacturers can create eco-friendly, bio-derived materials with enhanced performance-like excipients that improve solubility or control drug release naturally. Another fast-emerging opportunity is customized excipient design using AI-assisted formulation modeling, allowing precise combinations tailored to specific APIs.
Why Pharmaceutical Excipients Are the Next Frontier in Biopharma Innovation?
Pharmaceutical excipients are emerging as the next frontier in biopharma innovation, transforming from passive ingredients into strategic enablers of advanced drug delivery. Traditionally used to stabilize or bulk up formulations, excipients now play a pivotal role in enhancing bioavailability, controlling release rates, and improving patient compliance—especially for complex biologics and personalized therapies.
As biologic drugs, mRNA vaccines, and gene therapies grow in prominence, the demand for multifunctional, high-purity, and bio-derived excipients is accelerating. Innovations such as plant-based polymers, lipid nanoparticles, and AI-assisted formulation design are reshaping how drugs are developed and delivered. Furthermore, sustainability and clean-label requirements are driving manufacturers to invest in green chemistry and renewable excipient sources.
Regulatory Barriers Slow Innovation in the Advancements
The high cost and complexity of regulatory approvals for new excipients are anticipated to hinder market growth during the forecast period. Pharmaceutical companies are cautious about using novel materials because each one requires extensive testing, safety data, and compatibility studies. This slows down innovation and increases costs for manufacturers.
Explore the Full Report for Deeper Insights 👉 https://www.precedenceresearch.com/pharmaceutical-excipients-market
Pharmaceutical Excipients Market’s Regulatory Landscape: Global Regulations
|
Country / Region |
Regulatory Body |
Key Regulations |
Focus Areas |
Notable Notes |
|
United States |
Food and Drug Administration (FDA). |
GMP requirements (21 CFR Part 211) |
Drug product manufacturer's responsibility |
These agencies are ensuring drug safety in the country. |
|
European Union |
European Medicines Agency (EMA) |
Falsified Medicines Directive (2011/62/EU) |
Harmonized standards |
The EMA aims for consistent regulation across EU member states, using centralized procedures for marketing authorization and guidance documents.. |
|
China |
National Medical Products Administration (NMPA). |
"Drug Master File (DMF) System |
Stricter oversight |
China has uniquely high regulatory requirements for excipients, with mandatory registration for all non-exempt items. |
Market
Dynamics: Market
Drivers: 🔹 Surge in biologics, biosimilars, and personalized medicine
driving excipient innovation. 🔹 Growing demand for
oral solid dosage forms in chronic disease management. 🔹 Expansion of
clean-label, natural, and plant-based excipient adoption. Market
Restraints: 🔹 High regulatory
costs and lengthy approval timelines for new excipient types. 🔹 Limited
standardization in excipient compatibility testing for novel active pharmaceutical
ingredients. Opportunities: 🔹 AI-assisted
excipient design for precision drug formulation. 🔹 Growth in lipid nanoparticles
(LNPs) and polymer-based excipients for mRNA vaccines. 🔹 Increasing
outsourcing of excipient manufacturing to Asia Pacific and Eastern Europe. Challenges: 🔹 Raw material price
volatility. 🔹 Supply chain
dependency for critical excipient categories post-pandemic. Emerging
Trends in Pharmaceutical Excipients: 🔹 Integration of machine
learning in formulation design for predictive stability and performance
modeling. 🔹 Nanotechnology-based
excipients
improving solubility and targeted drug delivery. 🔹 Shift toward smart
excipients with dual or multifunctional roles (e.g., binder + stabilizer). 🔹 Development of 3D printing-compatible excipients for personalized dosage
manufacturing. 🔹 Adoption of green
chemistry and biodegradable excipients to meet ESG standards. Pharmaceutical
Excipients Market Perspective Report
Attributes Key Statistics Market Size in 2025 USD 9.31 Billion Market Size in 2025 USD 9.86 Billion Market Size by 2034 USD 15.43 Billion CAGR from 2025 to 2034 5.75% Largest Market North America Fastest Growing Market Europe Base Year 2024 Forecast Period 2025 to 2034 Segments Covered Functionality, Excipient Type, Regional Key Market Drivers Rising demand for advanced drug formulations, increasing
biologics production, and sustainable excipient innovation Key Market Restraints Stringent regulatory requirements and high R&D costs for new
excipients Opportunities Growth in bio-derived, plant-based, and multifunctional
excipients; AI-assisted formulation modeling Key Players BASF SE, Ashland Global, Evonik Industries AG, Roquette Frères,
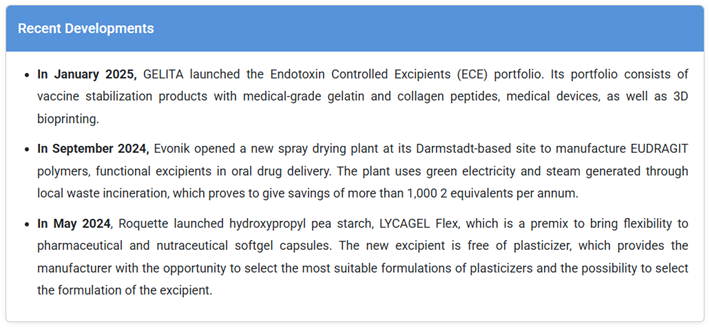
DuPont, Lubrizol Corporation Recent Developments Evonik’s new spray drying plant (2024); GELITA’s Endotoxin
Controlled Excipients launch (2025) Regional Insights North America leads; Europe growing fastest; Asia Pacific
emerging with strong pharma manufacturing base Research Methodology Top-down and bottom-up approaches, primary and secondary data
validation
Why Choose
This Report? ➢ This report comes with 12 months of
online access and quarterly update provisions. ➢ Get the latest report
version on request. ➡️ Become a valued research partner with
us ☎ https://www.precedenceresearch.com/schedule-meeting One
of the most notable industry examples underscoring innovation in the
pharmaceutical excipients market is Evonik Industries AG’s advancement in
functional polymer excipients. The company’s flagship EUDRAGIT® polymer
line demonstrates how excipient innovation is redefining modern drug
delivery. Challenge: Solution: Impact: Key
Takeaway: For
inquiries regarding discounts, bulk purchases, or customization requests,
please contact us at sales@precedenceresearch.com How
the Growth of the Global Pharmaceutical Market Accelerates the Pharmaceutical
Excipients Market Expanding
Pharmaceutical Market Underpins Excipient Demand According
to Precedence Research, the global pharmaceutical market size is projected to reach
around USD 2.87 trillion by 2034, growing from an estimated USD 1.64 trillion in 2024 at
a strong CAGR of 5.8% from 2025 to 2034. This exponential growth
directly strengthens the pharmaceutical excipients market, which is forecast to
rise from USD 9.31 billion in 2025 to USD 15.43 billion by 2034. The
correlation is clear — as pharmaceutical manufacturing, R&D spending, and
drug commercialization expand, the requirement for high-quality, functional,
and sustainable excipients intensifies proportionally. Drug
Innovation Drives the Need for Advanced Excipients The Complete Study is Now Available
for Immediate Access | Download the Sample Pages of this Report@ https://www.precedenceresearch.com/sample/6227 Massive
Drug Production and Generic Expansion Boost Consumption Sustainability
and Regulatory Alignment Strengthen Market Synergy Conclusion:
A Symbiotic Growth Path Between Pharmaceuticals and Excipients Don’t
Miss Out! | Instant Access to This Exclusive Report 👉 https://www.precedenceresearch.com/checkout/6227 Pharmaceutical
Excipients Market Key Regional Analysis: Strong
Pharmaceutical Infrastructure Positions North America at the Forefront? North
America held the dominant share of the pharmaceutical excipients market in
2024, owing to the presence of a stronger pharmaceutical infrastructure and access
to advanced formulation technologies in the current period. Furthermore, the United
States has major drug makers which is contributing to the industry graph in
recent years. What
is the United States Pharmaceutical Excipients Market Size? Precedence
Research predicts that, the U.S. pharmaceutical excipients market size is
projected to reach approximately USD 5.32 billion by 2034, increasing from USD
3.08 billion in 2025, with a solid CAGR of 6.26% from 2025 to 2034. The
U.S. represents one of the most mature and highly regulated pharmaceutical
excipients markets globally. Driven by advanced R&D capabilities, strong
demand for novel drug delivery systems, and a high concentration of innovative
drug manufacturers, the country shows strong demand for functional and
specialty excipients. Key
areas of focus include solubility enhancement, sustained release, and
biologics-supportive excipients, particularly for injectables and lipid-based
systems. Regulatory scrutiny from the FDA ensures that excipient manufacturers
maintain strict quality, safety, and traceability standards. Furthermore,
domestic production is increasingly being favored to reduce dependence on
international supply chains, particularly post-pandemic. What
Makes Asia Pacific a Magnet for Pharma Manufacturing? Asia
Pacific is expected to expand notably during the forecast period, owing to its enlarged
pharmaceutical production capacity and exceptional government support in recent
years. Also, the region has unique benefits like the presence of a skilled
workforce, low labor cost, and an enlarged base due to a huge population, which
has actively driven the regional growth over the past few years. India's
pharmaceutical excipients market is witnessing steady growth, driven by the
country's expanding generic drug manufacturing sector and its position as a
global pharmaceutical hub. The increasing domestic demand for affordable
medicines, alongside exports to regulated markets like the U.S. and Europe, is
encouraging the use of high-quality excipients. Indian manufacturers are
focusing on improving GMP compliance and adopting international pharmacopeial
standards to meet global quality benchmarks. Additionally,
there's a growing trend toward producing plant-based and clean-label
excipients, aligning with both domestic and international preferences.
Government initiatives supporting pharmaceutical infrastructure and bulk drug
parks are also positively impacting excipient production and localization. China's
pharmaceutical excipients market is growing rapidly, supported by the country's
expanding pharmaceutical manufacturing base and increasing domestic consumption
of medicines. As China strengthens its regulatory framework, there is rising
demand for high-purity, pharma-grade excipients that meet both Chinese and
international standards. Local companies are scaling up capabilities in
functional excipients and investing in R&D for customized solutions. Additionally,
government policies encouraging self-reliance in pharmaceutical ingredients are
promoting local excipient production. While historically focused on commodity
excipients, the market is gradually shifting toward value-added,
multifunctional, and clean-label products to meet evolving domestic and export
requirements. Pharmaceutical
Excipients Market Segmentation Analysis: Functionality
Insights: Why
Did Fillers and Diluents Segment Dominate the Market in 2024? The
fillers and diluents segment held the largest share of the pharmaceutical
excipients market in 2024, owing to its being considered the most essential
in almost every oral solid dosage form, such as tablets and capsules. Also, by
helping in adjusting drug size while improving compressibility, the filler and
diluent segment has gained major industry attention in recent years. Moreover,
the versatility of the fillers and diluents is likely to create lucrative
opportunities in the industry for the upcoming years. Excipient
Type: How
the Lactose-Based Excipients Segment Maintains Its Dominance in the Current
Industry? The
lactose-based
excipient segment
held the largest share of the market in 2024 due to its being considered the
most effective segment for delivering therapeutic genes in the cells. Moreover,
having unique properties such as targeting specific tissues and long-lasting
effects, the viral vectors have gained immense industry attention in recent
years, as per the current market survey. You can
place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 804
441 9344 Competitive
Landscape of Pharmaceutical Excipients Market: Top
Companies Company Headquarters Key
Offerings / Strengths Ashland
Global Holdings Wilmington,
Delaware, USA Offers a
broad portfolio of cellulose-based polymers (e.g., HPMC, CMC), film coatings,
binders, disintegrants, and controlled release agents. Strong in oral solid
dosage forms and biologics support. BASF SE Ludwigshafen,
Germany Provides a
wide range of pharmaceutical-grade excipients including polymers,
polyethylene glycols, and functional solubilizers. Focus on controlled
release and poorly soluble APIs. DuPont Wilmington,
Delaware, USA Supplies
functional polymers and resins used in taste masking and controlled release
applications across various dosage forms. Roquette
Frères Lestrem,
France Specializes
in plant-based excipients such as starch derivatives, sugars, and polyols.
Offers excipients for oral, injectable, and nutraceutical formulations. Evonik
Industries AG Essen /
Darmstadt, Germany Strong in
functional and custom polymers like EUDRAGIT®. Offers excipients for oral,
parenteral, and topical delivery. Also involved in lipid systems and LNPs. Associated
British Foods London, UK Focuses
primarily on food and nutritional ingredients. May contribute to excipient
markets via starches, sugars, and basic ingredients, though less
pharma-specific. Archer
Daniels Midland (ADM) Chicago,
Illinois, USA Supplies
plant-based raw materials like starches and sugars. Plays a role in the
excipient supply chain, particularly in basic feedstock materials. Lubrizol
Corporation Wickliffe,
Ohio, USA Known for
specialized excipients like Carbopol®, polycarbophil, and solubility
enhancers. Strong in injectables, controlled release, and formulation
support. Croda
International East
Yorkshire, UK Offers
natural and specialty chemical-based excipients including surfactants and
bio-based functional ingredients for drug delivery and formulation. Kerry
Group Tralee,
County Kerry, Ireland Provides
ingredients for health and nutrition sectors. Involved in stabilizers,
emulsifiers, and basic excipient functions, particularly for nutraceuticals.
What
is Going Around the Globe? The
global pharmaceutical excipients market is witnessing notable developments as
leading manufacturers introduce advanced, sustainable, and high-performance
excipients to meet evolving drug formulation needs. Additional
Developments Across the Industry: 🔹 Ashland Global
Holdings
is expanding its range of hypromellose-based coatings for
sustained-release oral dosage forms to improve patient compliance. 🔹 BASF SE is investing in
next-generation solubilizers and binders optimized for poorly soluble
APIs to address bioavailability challenges in modern therapeutics. 🔹 Lubrizol Corporation is enhancing its Carbopol®
platform for injectables, offering higher purity and consistency for use in
biologic formulations. 🔹 Croda International is advancing its bio-based
surfactants and emulsifiers aimed at improving solubility and absorption in
lipid nanoparticles (LNPs) and mRNA delivery systems. 🔹 DuPont is focusing on controlled-release
excipient technology tailored for personalized medicine and micro-dose
formulations. Pharmaceutical
Excipients Market Segmentation: By
Functionality 🔹Fillers and Diluents 🔹Suspending and Viscosity Agents 🔹Coating Agents 🔹Binders 🔹Disintegrants 🔹Colorants 🔹Lubricants and Glidants 🔹Preservatives 🔹Emulsifying Agents 🔹Flavoring Agents and Sweeteners 🔹Other Functionalities By
Excipient Type 🔹 Lactose-based Excipients • α-lactose monohydrate •
Anhydrous
α-lactose •
Anhydrous
β-lactose •
Amorphous
Lactose 🔹 Cellulose-based •
Microcrystalline
Cellulose (MCC) •
Cellulose
Ethers •
Others 🔹 Starches 🔹 Carboxymethylcellulose Sodium (CCS) 🔹 Sodium Starch Glycolate (SSG) 🔹 Fine Chemicals 🔹 Mannitol 🔹 Biopharma Excipients 🔹 Others By Regions 🔹 North America 🔹 Europe 🔹 Asia Pacific 🔹Latin America 🔹Middle East & Africa (MEA) Thanks for reading you can also get individual
chapter-wise sections or region-wise report versions such as North America,
Europe, or Asia Pacific. Don’t Miss Out! | Instant Access to This
Exclusive Report 👉 https://www.precedenceresearch.com/checkout/1761 Pharmaceutical
Excipients Market — FAQs ✚ What is the market size
and growth outlook? ✚ What drives this market’s
growth? ✚ What restrains market
expansion? ✚ Which regions matter most? ✚ Which functionality
segment leads today? ✚ Which functionality will
grow fastest? ✚ Which excipient types
dominate? ✚ How does broader pharma
growth affect excipients? ✚ What innovation themes
define the next cycle? ✚ What are key
opportunities? ✚ How are sustainability
goals impacting suppliers? ✚ Which companies are
prominent? Stay
Ahead with Precedence Research Subscriptions Unlock
exclusive access to powerful market intelligence, real-time data, and
forward-looking insights, tailored to your business. From trend tracking to
competitive analysis, our subscription plans keep you informed, agile, and
ahead of the curve. Browse Our
Subscription Plans@ https://www.precedenceresearch.com/get-a-subscription About Us Precedence
Research is a global market intelligence and consulting powerhouse, dedicated
to unlocking deep strategic insights that drive innovation and transformation.
With a laser focus on the dynamic world of
life sciences, we
specialize in decoding the complexities of cell and
gene therapy,
drug development, and oncology markets, helping our clients stay ahead in
some of the most cutting-edge and high-stakes domains in healthcare. Our
expertise spans across the biotech and pharmaceutical ecosystem, serving
innovators, investors, and institutions that are redefining what’s possible in regenerative medicine, cancer care, precision therapeutics,
and beyond. Web: https://www.precedenceresearch.com Our
Trusted Data Partners: Towards Healthcare | Nova One Advisor Get Recent
News 👉 https://www.precedenceresearch.com/news For Latest
Update Follow Us: ✚ Related Topics You May Find Useful: ➡️ Nutraceutical
Excipients Market: Explore how functional
food innovation and clean-label nutrition are shaping the future of
nutraceutical formulations ➡️ High
Potency Active Pharmaceutical Ingredients (HPAPI) Market: Examine the rise of
targeted therapies and precision oncology driving HPAPI demand globally ➡️ Pharmaceutical
Intermediates Market: Uncover how growing API
production and outsourcing trends are fueling intermediate manufacturing ➡️ Pharmaceutical
Chemicals Market: Learn how innovation in
fine chemicals and green synthesis supports modern drug development ➡️ Pharmaceutical
Quality Control Market:
Understand how digital validation and AI-based testing are transforming
pharmaceutical quality standards ➡️ Drug Formulation Market: See how advanced
formulation science and novel delivery systems are revolutionizing therapeutic
effectiveness ➡️ Pharmaceutical
Impurity Synthesis and Isolation Services Market: Discover how regulatory
pressure and analytical precision are driving demand for impurity profiling
services ➡️ Pharmaceutical
Stability and Storage Services Market: Analyze how temperature-controlled
logistics and stability testing ensure long-term drug efficacy ➡️ Generic Drugs Market: Track how affordability,
patent expirations, and healthcare reforms are accelerating the global generics
boom
Case
Study: Evonik’s EUDRAGIT® Polymers Revolutionize Oral Drug Delivery
Pharmaceutical companies face a growing need to enhance the solubility,
bioavailability, and targeted release of poorly soluble active pharmaceutical
ingredients (APIs). Traditional excipients often fail to maintain consistent
performance in complex formulations, leading to inefficiencies and higher
development costs.
Evonik developed a new generation of EUDRAGIT® polymers designed for controlled
and sustained drug release in oral solid dosage forms. In September 2024,
the company opened a state-of-the-art spray drying plant in Darmstadt,
Germany, specifically dedicated to producing these polymers under
sustainable conditions. The plant operates using green electricity and steam
sourced from local waste incineration, achieving an estimated reduction
of over 1,000 tons of CO₂ annually.
This innovation not only enhances excipient performance and stability but also
aligns with the pharmaceutical industry’s growing emphasis on eco-friendly
and regulatory-compliant excipient manufacturing. The project demonstrates
how sustainability and advanced functionality can coexist in excipient design, setting
a benchmark for the future of drug formulation.
Evonik’s approach highlights a larger industry trend — the integration of
sustainability, precision engineering, and functional design in excipient
innovation. As regulatory pressures rise and biologics gain prominence, such
multifunctional excipients are becoming essential for ensuring both therapeutic
efficacy and environmental responsibility.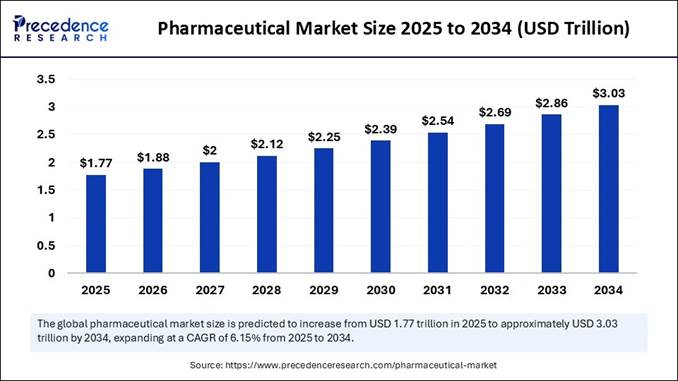
The surge in novel drug formulations, biologics, biosimilars, and gene
therapies has transformed excipients from passive ingredients into critical
enablers of drug performance. As pharmaceutical companies innovate new delivery
mechanisms and biologic molecules, they increasingly depend on multifunctional
excipients that enhance bioavailability, control drug release, and improve
patient adherence. This ongoing innovation in the global pharmaceutical
industry directly drives demand for high-purity and bio-derived excipients that
meet evolving regulatory and therapeutic standards.
Rising global demand for affordable medicines and generic drugs, especially in Asia
Pacific, Latin America, and Eastern Europe, is rapidly increasing excipient
consumption. India and China — leading producers of generics — are scaling
manufacturing to meet international export demands, further expanding the
excipients market. The pharmaceutical production surge across emerging
markets creates consistent, high-volume demand for essential excipients like
fillers, binders, and diluents, reinforcing global market growth.
The pharmaceutical industry’s pivot toward eco-friendly, compliant, and
sustainable production models is reshaping excipient innovation.
Manufacturers are investing in bio-derived and plant-based excipients to
align with clean-label and environmental standards. Simultaneously, stricter
regulatory oversight from agencies such as the FDA, EMA, and NMPA ensures that
excipient suppliers maintain top-tier quality and traceability. This alignment
between pharmaceutical regulation and excipient innovation creates a mutually
reinforcing cycle that sustains long-term market expansion.
The pharmaceutical excipients market’s forecasted value of USD 15.43 billion
by 2034 is inseparable from the broader pharmaceutical industry’s
transformation. As global drug pipelines diversify and new therapeutic classes
emerge, excipients are evolving into strategic components of modern drug
development. The synergy between the pharmaceutical and excipient markets
ensures that innovation in one continually fuels advancement in the other —
solidifying excipients as indispensable pillars of the global pharmaceutical
ecosystem.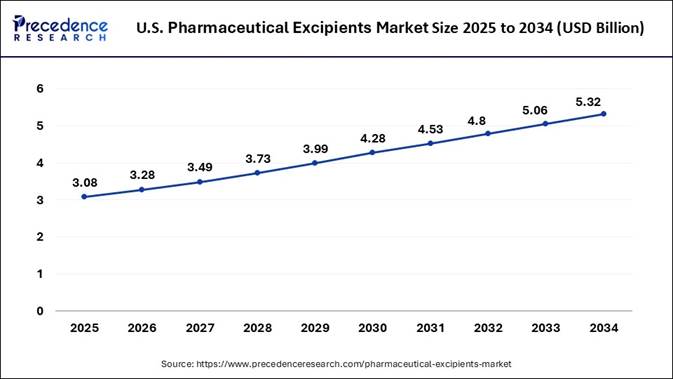
The Full Study is Readily Available | Download the Sample Pages of this Report@ https://www.precedenceresearch.com/sample/1761United States Pharmaceutical Excipients Market Analysis
India Pharmaceutical Excipients Market Analysis
China Pharmaceutical Excipients Market Analysis
➡️ The market is valued at USD 9.31
billion in 2025 and is projected to reach USD 15.43 billion by 2034, at a
2025–2034 CAGR of 5.75%.
➡️ Rising demand for advanced drug
formulations, the scale-up of biologics and biosimilars, and increasing
adoption of bio-derived, sustainable excipients.
➡️ High regulatory costs, lengthy
approval timelines for new excipients, and inconsistent compatibility standards
with novel APIs.
➡️ North America leads in 2024; Europe is
the fastest-growing region; Asia Pacific is expanding rapidly on the back of
generics manufacturing.
➡️ Fillers and diluents hold the largest
share in 2024, reflecting ubiquity in oral solid dosage manufacturing.
➡️ Coating agents are expected to post
significant growth over the forecast period, supported by controlled-release
and taste-masking needs.
➡️ Lactose-based excipients hold the
highest 2024 share; cellulose-based excipients show notable growth through
2034.
➡️ As global pharma scales R&D,
launches, and manufacturing, excipient demand rises proportionally for volume,
quality, and specialized performance.
➡️ Multifunctional/smart excipients,
plant-based and clean-label inputs, AI-assisted formulation design,
nanotechnology carriers, and 3D-printing-ready grades.
➡️ AI-guided excipient design, lipid
nanoparticles and polymer systems for mRNA/biologics, and contract
manufacturing/outsourcing in Asia Pacific and Eastern Europe.
➡️ Green chemistry, renewable feedstocks,
and eco-efficient manufacturing (e.g., reduced CO₂ operations) are becoming
selection criteria alongside performance.
➡️ BASF SE, Ashland Global, Evonik
Industries, Roquette Frères, DuPont, Lubrizol, Croda, ADM, Kerry Group, and
others across polymers, solubilizers, and plant-based portfolios.
______________________________________________________________________________

