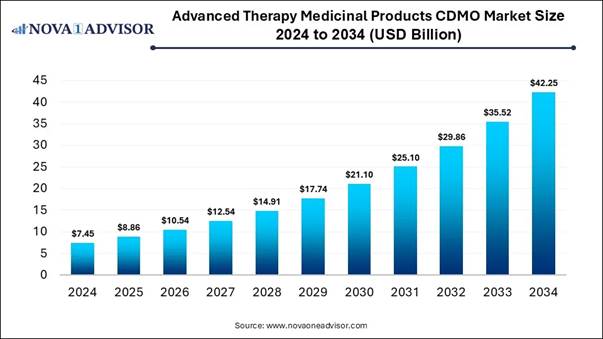
According to Nova One Advisor, the global advanced therapy medicinal products CDMO market size was estimated at USD7.45 billion in 2024 and is projected to grow to USD 42.25 billion by 2034, rising at a compound annual growth rate (CAGR) of 18.95% from 2025 to 2034.
Key Takeaways
⬥︎North America held a major share of the market in 2024.
⬥︎Asia Pacific is expected to grow at the fastest CAGR in the studied years.
⬥︎By product, the gene therapy segment led the advanced therapy medicinal products CDMO market in 2024.
⬥︎By product, the cell therapy segment is expected to grow notably during 2025-2034.
⬥︎By phase, the phase II segment registered dominance in the market in 2024.
⬥︎By phase, the phase III segment is expected to witness rapid expansion in the coming years.
⬥︎By indication, the oncology segment registered dominance in the market in 2024.
⬥︎By indication, the neurological and genetic disorders segment is expected to witness rapid expansion in the coming years.
The Complete Study is Now Available for
Immediate Access | Download the Sample Pages of this Report@ https://www.novaoneadvisor.com/report/sample/8439
What is an Advanced Therapy Medicinal
Products CDMO? The global advanced
therapy medicinal products CDMO market mainly refers to the contract
development and manufacturing organizations that offer specialized, end-to-end
services for complex gene, cell, and tissue-based therapies. Key advantages for
pharmaceutical
and biotech companies include accessing specialized manufacturing facilities,
ensuring regulatory compliance, and accelerating time-to-market without
significant capital investment. The market is driven by the increasing
prevalence of rare diseases, a growing pipeline of cell
and gene therapies in clinical
trials, and advancements in gene-editing
technologies like CRISPR. What are the Key Drivers in the Advanced
Therapy Medicinal Products CDMO Market? A prominent driver is the rising number of
clinical trials, innovation in gene-editing tools like CRISPR-Cas9, advanced
bioprocessing techniques, and the integration of automation and artificial
intelligence, which are improving manufacturing efficiency,
scalability, and quality control. The increasingly significant investment and
grants in ATMP research are accelerating the development of new therapies and
creating opportunities for market expansion. Increasing personalized medicine
and growth of cell
and gene therapy pipelines. You can place an order or ask any
questions, please feel free to contact at sales@novaoneadvisor.com |
+1 804 441 9344 Advanced Therapy Medicinal Products CDMO
Market Report Scope Report Attribute Details Market size value in 2025 USD 8.86 billion Revenue forecast in 2034 USD 42.25 billion Growth rate CAGR of 18.95% from 2025 to 2034 Base year for estimation 2024 Historical data 2018 - 2024 Forecast period 2025 - 2034 Quantitative units Revenue in USD million/billion and CAGR
from 2025 to 2034 Report coverage Revenue forecast, company ranking,
competitive landscape, growth factors, and trends Segments covered Product, Phase, Indication, Region Regional scope North America; Europe; Asia Pacific;
Latin America; MEA Country scope U.S.; Canada; UK; Germany; France; Italy;
Spain; Denmark; Sweden; Norway; Japan; China; India; Australia; Thailand;
South Korea; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE;
Kuwait Key companies profiled Celonic; Bio Elpida; CGT Catapult;
Rentschler Biopharma SE; AGC Biologics; Catalent; Lonza; WuXi Advanced
Therapies; BlueReg; Minaris Regenerative Medicine; Patheon Customization scope Free report customization (equivalent up
to 8 analyst’s working days) with purchase. Addition or alteration to
country, regional & segment scope.
Buy Now Full Report: https://www.novaoneadvisor.com/report/checkout/8439
What Are the Growing Trends Associated
with the CDMO Services for Pharma and Biotech Market? ⬥︎ Rise of advanced and specialised therapies: The growing complexity of new drugs, such
as antibody-drug conjugates, oligonucleotides, and cell and gene therapies, is
driving demand for CDMOs with highly specialised expertise and technology. ⬥︎ Demand for personalised medicine: The move towards tailored treatments
requires flexible manufacturing processes that many CDMOs are developing, often
using automation and modular systems to accommodate varying batch sizes and
patient-specific needs. ⬥︎ Globalisation and supply chain complexity Pharmaceutical companies are increasingly
relying on CDMOs to navigate complex global supply chains and ensure consistent
product quality across different markets. ⬥︎ Specialization There is a growing trend of CDMOs
specialising in specific therapeutic areas or manufacturing processes, allowing
them to offer deep expertise in a particular niche. ⬥︎ Growth of outsourcing by smaller companies: Smaller and mid-sized pharmaceutical
companies are increasingly using CDMOs to avoid large capital investments in
manufacturing facilities, which is a key growth driver for the CDMO market. What are the Major Trends in the
Advanced Therapy Medicinal Products CDMO Market? 🔹In May 2024, Catalent and Siren Biotechnology, the companies formed
a strategic agreement to advance and manufacture Siren Biotechnology's AAV
immuno-gene treatments for cancer. 🔹In December 2024, Novo Holdings acquired Catalent, a major
acquisition (USD 16.5 billion), creating a multi-modal manufacturing giant,
significantly consolidating capacity in the biomanufacturing space. What is the Emerging Challenge in the
Advanced Therapy Medicinal Products CDMO Market? The market is mainly facing a hurdle in
ATMPs involve complex, often patient-specific (autologous) processes using
living cells or viral vectors, which are difficult to scale up from small-scale
clinical production to large-scale commercial manufacturing while maintaining
product consistency and quality. High cost of affordability and shortage of
proficient and experienced personnel in the manufacturing of cell and gene
therapies. Regional Analysis How did North America Dominate the
Market in 2024? In 2024, North America captured the biggest
revenue share of the market. The robust ecosystem of scientific innovation and
significant investment. The United States, in particular, led with a high
concentration of specialized biotechnology
firms and extensive R&D activity. Supportive regulatory pathways from the
FDA, coupled with a large number of clinical
trials, accelerated the development and commercialization of new
therapies. 🔹For instance, In March 2024, Alcami Corporation collaborated with
Tanvex CDMO to provide integrated services. Large CDMOs like Thermo Fisher have
also acquired smaller, niche players to expand their ATMP capabilities. United States Advanced Therapy Medicinal
Products CDMO Market: The United States is experiencing increased
M&A activity among CDMOs, growing adoption of advanced digital
manufacturing, and strategic partnerships to secure domestic manufacturing
capacity amid supply chain concerns. A booming pipeline of complex cell and
gene therapies and strong R&D investment. This growth is supported by
favorable FDA regulations, like accelerated approvals and a shift towards more
scalable allogeneic therapies. Why did Asia Pacific Grow Notably in the
Market in 2024? Asia Pacific is anticipated to expand at a
rapid CAGR during 2025-2034 in the advanced therapy medicinal products CDMO
market. The strong government support and a cost-effective manufacturing
ecosystem. Favourable regulatory pathways in countries like China and Australia
attracted increased R&D and clinical trial activity. Substantial regional
investment and expanding infrastructure solidified the region as a competitive
and attractive hub for global biopharmaceutical
companies seeking efficient ATMP production. 🔹For instance, In October 2024, Samsung Biologics, a
major CDMO, continued its significant expansion in South Korea. It
announced a record $1.24 billion manufacturing deal with an Asian
pharmaceutical company. China Advanced Therapy Medicinal
Products CDMO Market: China's advanced therapy medicinal products
CDMO market is rapidly expanding due to a surge in clinical trials and strong
government support through favourable regulations. A boom in domestic biotech
firms, particularly in cell therapy for oncology, is driving significant demand
for specialized manufacturing services. Despite rapid growth, geopolitical
tensions, such as the U.S. BIOSECURE Act, are influencing supply chain
strategies. Immediate Delivery Available, Get Full
Access @ https://www.novaoneadvisor.com/report/checkout/8439
Segmental Insights By product analysis Which Product Led the Advanced Therapy
Medicinal Products CDMO Market in 2024? The gene
therapy CDMO services segment accounted for a dominant share of the
market in 2024. The technical complexity and high demand for viral vector
manufacturing, a process often outsourced to specialized CDMOs. Innovations in
gene editing technologies like CRISPR, combined with increasing R&D for
rare genetic disorders, ensure the gene
therapy segment maintains a significant and influential role in market
dynamics. 🔹In October 2024, Forge Biologics, a member of Ajinomoto Bio-Pharma
Services, launched its FUEL manufacturing platform specifically designed for
high-yield AAV production to accelerate gene therapy development. However, the cell
therapy segment is predicted to expand at a lucrative CAGR. The
commercial success of CAR-T treatments and a pipeline expanding into new
therapeutic areas. The industry-wide shift towards scalable, cost-effective
allogeneic "off-the-shelf" therapies, which CDMOs are increasingly
capable of manufacturing efficiently. Adoption of advanced automation and
closed-system manufacturing, making outsourcing an attractive option for
numerous small and mid-sized biotech firms. By phase analysis What Made the Phase II Segment Dominant
in the Market in 2024? The phase II segment held a major revenue
share of the advanced therapy medicinal products CDMO market in 2024. A high
volume of successful early-stage candidates transitioning to larger efficacy
trials. This phase necessitates crucial manufacturing scale-up, process
optimization, and adherence to complex GMP standards, driving a high demand for
specialized CDMO expertise and infrastructure. On the other hand, the phase III segment is
estimated to expand rapidly in the predicted timeframe. The high volume of
therapies is advancing into pivotal trials and preparing for commercial launch.
This phase demands significantly larger-scale production and stringent
regulatory validation, requirements that often exceed the in-house capabilities
of many biotech firms. Consequently, developers rely heavily on the specialized
expertise and capacity of CDMOs to manage manufacturing complexities and
navigate the final regulatory hurdles efficiently. By indication analysis What Made the Oncology Segment Dominant
in the Market in 2024? The oncology
segment held a major revenue share of the advanced therapy medicinal products
CDMO market in 2024. The intense clinical trial activity and substantial
investment in cancer-related advanced therapies. The unique potential and high
unmet medical need for treatments like CAR-T and TCR therapies drove
significant R&D efforts. Manufacturing these complex immunotherapies requires
specialized expertise and infrastructure, leading biotech firms to heavily rely
on expert CDMO partners. On the other hand, the neurological and
genetic disorders segment is estimated to expand rapidly in the predicted
timeframe. The vast unmet medical need and technological breakthroughs in gene
editing. The increasing number of clinical trials and regulatory support for
orphan drugs is accelerating the development pipeline for these complex
conditions. The biopharma companies are heavily relying on specialized CDMOs
for the development and manufacturing expertise required for these advanced,
often curative, therapies. Top Key Players in Advanced Therapy
Medicinal Products CDMO Market: ⬥︎Celonic GmbH – Provides end-to-end
development and GMP manufacturing services for cell and gene therapies, with
expertise in viral vector and biologics production. ⬥︎Bio Elpida – Specializes in small-batch
GMP manufacturing for cell and gene therapies, focusing on process development
and aseptic fill-finish for early clinical stages. ⬥︎Cell and Gene Therapy Catapult (CGT Catapult) – A UK-based innovation and manufacturing center supporting
technology transfer, process optimization, and scale-up of advanced therapies. ⬥︎Rentschler Biopharma SE – Offers
full-service biopharmaceutical development and GMP manufacturing, including
viral vector and gene therapy production capabilities. ⬥︎AGC Biologics – Provides integrated
development, manufacturing, and analytical services for viral vectors, plasmid
DNA, and cell therapies, serving both clinical and commercial stages. ⬥︎Catalent, Inc. – A leading global CDMO
offering viral vector manufacturing, plasmid DNA production, and advanced
therapy fill-finish services through its Cell & Gene Therapy segment. ⬥︎Lonza Group Ltd. – Delivers comprehensive
solutions for cell and gene therapy development and manufacturing, including
viral vector platforms and commercial-scale production. ⬥︎WuXi Advanced Therapies (WuXi AppTec Group) – Provides integrated cell and gene therapy CDMO services, including
process development, testing, and GMP manufacturing for viral vectors and cell
therapies. ⬥︎BlueReg Group – Offers specialized
regulatory consulting and strategy support for ATMP developers, focusing on
market access, compliance, and product lifecycle management. ⬥︎Minaris Regenerative Medicine – Focuses
on global contract development and manufacturing of cell and gene therapies,
with expertise in autologous and allogeneic manufacturing solutions. What are the Revolutionary Developments
in the Advanced Therapy Medicinal Products CDMO Market? 🔹In March 2024, Alcami Corporation partnered with Tanvex CDMO to
provide clients with a complete solution from bulk drug substance production to
finished drug products. 🔹In January 2024, Pluri Inc. created its PluriCDMO business division
to offer specialized expertise and access to its GMP cell therapy production
facility. 🔹In October 2024, Forge Biologics (a member of Ajinomoto Bio-Pharma
Services) launched its proprietary FUEL platform in October 2024 to accelerate
AAV gene therapy manufacturing Related Report ⬥︎ Active Pharmaceutical Ingredients CDMO Market - The global active
pharmaceutical ingredients CDMO market size was valued at USD 129.25
billion in 2024 and is anticipated to reach around USD 273.92 billion by 2034,
growing at a CAGR of 7.8% from 2025 to 2034. ⬥︎ Biopharmaceutical CDMO Market - The
global biopharmaceutical
CDMO market was valued at USD 21.15 billion in 2024 and is projected to
hit around USD 49.61 billion by 2034, expanding at a CAGR of 8.9% during the
forecast period of 2025 to 2034. ⬥︎ Biologics CDMO Market - The global biologics
CDMO market size is calculated at USD 22.45 billion in 2024, grows to
USD 25.92 billion in 2025, and is projected to reach around USD 94.60 billion
by 2034, growing at a solid CAGR of 15.47% from 2025 to 2034. ⬥︎ Oligonucleotide CDMO Market - The
global oligonucleotide
CDMO market size is calculated at USD 3.15 billion in 2024, grows to
USD 3.84 billion in 2025, and is projected to reach around USD 22.73 billion by
2034, at a compound annual growth rate (CAGR) of 21.85% from 2024 to 2034. ⬥︎ Cell And Gene Therapy CDMO Market - The
global Cell
And Gene Therapy CDMO market size was estimated at USD 7.52 billion in
2024 and is projected to hit around USD 88,84 billion by 2034, growing at a
CAGR of 28.1% during the forecast period from 2025 to 2034. ⬥︎ U.S. Active Pharmaceutical Ingredients CDMO Market - The U.S.
active pharmaceutical ingredients CDMO market size is calculated at USD
25.85 billion in 2024, grows to USD 27.22 billion in 2025, and is projected to
reach around USD 43.33 billion by 2034, growing at a CAGR of 5.3% from 2025 to
2034. ⬥︎ North America Topical Drugs CDMO Market - The North
America topical drugs CDMO market size was exhibited at USD 15.25
billion in 2024 and is projected to hit around USD 42.87 billion by 2034,
growing at a CAGR of 10.89% during the forecast period 2025 to 2034. ⬥︎ Topical Drugs CDMO - The topical
drugs CDMO market size was exhibited at USD 46.15 billion in 2024 and
is projected to hit around USD 137.06 billion by 2034, growing at a CAGR of
11.5% during the forecast period 2024 to 2034. ⬥︎ U.S. Pharmaceutical CDMO Market - The U.S.
pharmaceutical CDMO market size was exhibited at USD 40.65 billion in
2024 and is projected to hit around USD 83.86 billion by 2034, growing at a
CAGR of 7.51% during the forecast period 2024 to 2034. ⬥︎ Veterinary CRO and CDMO Market - The Veterinary
CRO and CDMO market size was exhibited at USD 7.15 billion in 2024 and
is projected to hit around USD 17.56 billion by 2034, growing at a CAGR of 9.4%
during the forecast period 2025 to 2034. ⬥︎ U.S. Small Molecule Innovator API CDMO Market - The U.S.
small molecule innovator API CDMO market size was exhibited at USD 8.50
billion in 2023 and is projected to hit around USD 15.48 billion by 2033,
growing at a CAGR of 6.18% during the forecast period 2024 to 2033. Segments Covered in the Report This report forecasts revenue growth at
country levels and provides an analysis of the latest industry trends in each
of the sub-segments from 2021 to 2034. For this study, Nova one advisor, Inc.
has segmented the advanced therapy medicinal products cdmo market. By Product • Gene Therapy • Cell Therapy • Tissue Engineered • Others By Phase • Phase I • Phase II • Phase III • Phase IV By Indication • Oncology • Cardiology • Central nervous system • Musculoskeletal • Infectious disease • Dermatology • Endocrine, metabolic, genetic • Immunology & inflammation • Ophthalmology • Hematology • Gastroenterology • Others By Region • North America • Europe • Asia-Pacific • Latin America • Middle East & Africa (MEA) Immediate Delivery
Available | Buy This Premium Research https://www.novaoneadvisor.com/report/checkout/8439 About-Us Nova One Advisor is a global leader
in market intelligence and strategic consulting, committed to delivering deep,
data-driven insights that power innovation and transformation across industries.
With a sharp focus on the evolving landscape of life sciences, we specialize in
navigating the complexities of cell and gene therapy, drug development, and the
oncology market, enabling our clients to lead in some of the most revolutionary
and high-impact areas of healthcare. Our expertise spans the entire
biotech and pharmaceutical value chain, empowering startups, global
enterprises, investors, and research institutions that are pioneering the next
generation of therapies in regenerative medicine, oncology, and precision medicine.
Web: https://www.novaoneadvisor.com/ Contact Us USA: +1 804 420 9370 Email: sales@novaoneadvisor.com For Latest Update Follow Us: LinkedIn Towards Healthcare | Towards Packaging | Towards Automotive | Towards Chem and Materials | Towards FnB | Towards Consumer Goods | Statifacts | Towards EV Solutions | Towards Dental | Market Stats Insight | Nutraceuticals Func Foods | Onco Quant | Sustainability Quant | Specialty Chemicals
Analytics

