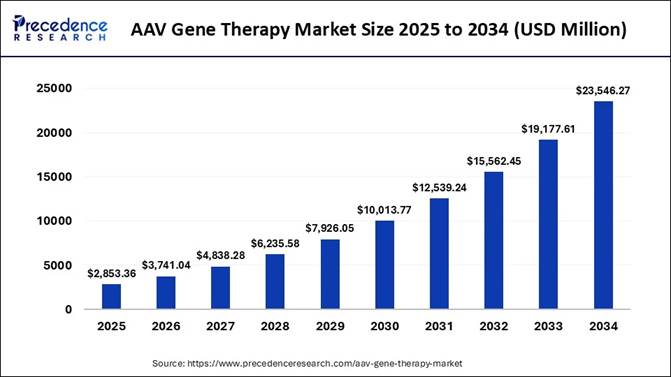
The global AAV gene therapy market size is expected to be worth USD 23,546.27 million by 2034, driven by rising approvals, expanding therapeutic applications, and increasing investments in advanced genetic treatment innovations.
According to Precedence Research, the global AAV gene therapy market size is calculated at USD 2,853.36 million in 2025 and is expected to grow from USD 3,741.04 million in 2026 to nearly USD 23,546.27 million by 2034. In terms of CAGR, the market is expected to expand at a healthy compound annual growth rate (CAGR) of 26.43% from 2025 to 2034.
AAV Gene Therapy Market Highlights:
🔹 North America dominated the
global AAV gene therapy market with a 42.19% share in 2024.
🔹
Asia Pacific is projected to grow at the fastest CAGR between 2025 and 2034.
🔹
Neurological disorders led the therapeutic area segment with a 29.4% share in
2024.
🔹
Muscular disorders are expected to record the fastest CAGR through the forecast
period.
🔹
AAV9 was the leading vector serotype, contributing 27.60% of the market in
2024.
🔹
Engineered / synthetic / hybrid capsids are anticipated to grow at a notable
CAGR over the projected period.
🔹
Intravenous (I.V.) administration held the highest share at 36.80% in 2024.
🔹
Intrathecal (I.T.) administration is expected to grow at a notable CAGR during
the forecast period.
🔹
Clinical therapies captured the largest share at 48.7% within the application
stage in 2024.
🔹
Commercialized therapies are projected to expand at a notable CAGR over the
forecast period.
🔹
In-house manufacturing accounted for 54.1% of the market in 2024.
🔹
CDMOs / vector production facilities are expected to grow at a notable CAGR
over the projected period.
🔹
Pharmaceutical and biotechnology companies generated the highest end-user share
at 52.8% in 2024.
🔹 Contract
research organizations (CROs) are projected to expand at a
notable CAGR during the forecast period.
AAV Gene Therapy Market Overview
and Industry Potential
The adeno-associated virus (AAV) gene therapy market is poised for transformative growth as demand accelerates for safer, more stable, and more efficient gene-delivery technologies. AAV vectors have become the preferred platform for numerous gene therapy programs because they offer a strong balance of safety, tissue specificity, and long-term expression.
As gene therapy shifts from experimental to clinically validated treatment options, AAVs are increasingly being incorporated into therapeutic pipelines addressing both rare and common diseases. Advancements in vector engineering, improved manufacturing systems, and rising investments from biotechnology and pharmaceutical companies further strengthen the commercial potential of this market. With a growing number of late-stage clinical trials and increasing regulatory support, the AAV gene therapy space is expected to expand rapidly, reshaping the future of genetic medicine.
Dive Into the Full Evidence-Backed Market Blueprint, Visit
Here 👉https://www.precedenceresearch.com/aav-gene-therapy-market
AAV
Gene Therapy Market Opportunity The
greatest growth potential for AAV gene therapy lies in its expanding use for
rare and ultra-rare diseases, where traditional treatments are limited or
nonexistent. Many of these conditions stem from a single genetic defect, making
them ideal candidates for AAV-mediated correction. As a result, biotech
companies are aggressively pursuing regulatory approvals for AAV-based
therapies, aiming to secure first-mover advantage in underserved therapeutic
areas.
Regulatory agencies increasingly encourage innovation in rare disease
treatments through accelerated approval pathways, orphan drug designations, and
financial incentives, further boosting the market’s attractiveness. As several
AAV therapies progress through late-stage development and approach
commercialization, they are expected to unlock substantial revenue
opportunities and draw significant attention as next-generation therapeutic
breakthroughs. Restraint:
High Production Cost and Manufacturing Complexity Despite
strong growth prospects, the AAV gene therapy market faces substantial barriers
due to the complexity and cost of vector manufacturing. Producing AAV at
clinical-grade quality requires highly controlled environments,
state-of-the-art bioreactors, stringent purification processes, and extensive
quality testing to ensure safety and consistency. These requirements
significantly increase production costs, posing challenges for both emerging
companies and large-scale manufacturers. Additionally,
the industry suffers from limited availability of skilled personnel with
expertise in gene vector development and GMP manufacturing. The combined burden
of specialized infrastructure, long production timelines, and stringent
regulatory requirements may slow market expansion and hinder new entrants from
competing effectively. Until scalable, cost-efficient manufacturing
technologies are widely adopted, production constraints will remain a notable
restraint on industry growth. ➡️
Become a valued
research partner with us ☎ https://www.precedenceresearch.com/schedule-meeting AAV Gene Therapy Market Scope and Strategic
Coverage
|
Report Attributes |
Key Details |
|
Market Size in 2025 |
USD 2,853.36 Million |
|
Market Size in 2026 |
USD 3,741.04 Million |
|
Market Size by 2034 |
USD 23,546.27 Million |
|
Growth Rate (2025–2034) |
CAGR of 26.43% |
|
Dominating Region (2024) |
North America (42.19% market share) |
|
Fastest-Growing Region |
Asia Pacific (Strongest CAGR through 2034) |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2034 |
|
Market Drivers |
Rising FDA approvals, expanding gene therapy pipelines, and increased investment in advanced vector engineering |
|
Market Challenges |
High manufacturing costs, GMP capacity shortages, and limited large-scale vector production infrastructure |
|
Market Opportunities |
Growing demand for rare disease therapies, rapid expansion of AAV-based CNS and muscular disorder treatments, and advancements in synthetic/engineered capsids |
|
Leading Vector Serotype (2024) |
AAV9 (27.60% share) |
|
Fastest-Emerging Vector Type |
Engineered/Synthetic/Hybrid AAV Capsids |
|
Top Route of Administration (2024) |
Intravenous (I.V.) – 36.80% share |
|
High-Growth Delivery Route |
Intrathecal (I.T.) for CNS gene delivery |
|
Largest Application Stage (2024) |
Clinical Therapies (48.7% share) |
|
Fastest-Growing Application Stage |
Commercialized Therapies |
|
Leading Manufacturing Type (2024) |
In-house Manufacturing (54.1% share) |
|
Fastest-Growing Manufacturing Type |
CDMOs / Vector Production Facilities |
|
Top End User (2024) |
Pharmaceutical & Biotechnology Companies (52.8% share) |
|
Fastest-Growing End User |
Contract Research Organizations (CROs) |
|
Segments Covered |
Therapeutic Area, Vector Serotype, Route of Administration, Application Stage, Manufacturing Type, End-User, Region |
|
Regions Covered |
North America, Europe, Asia-Pacific, Latin America, Middle East & Africa |
|
Key Clinical Focus Areas |
Neurological Disorders, Muscular Disorders, Ophthalmic Diseases, Hematologic Conditions, Metabolic Disorders |
|
Regulatory Tailwinds |
Orphan drug designations, accelerated approvals, and supportive frameworks for rare disease gene therapies |
|
Commercial Momentum |
Increasing approvals of AAV therapies (e.g., Zolgensma, Luxturna) and expanding late-stage pipelines in CNS and muscular diseases |
|
Technological Advancement |
Rapid evolution of AI-driven vector design, improved tropism engineering, and scalable bioreactor-based AAV production |
Immediate Delivery Available | Buy This Premium Research Report@
https://www.precedenceresearch.com/checkout/6873 How the Global Gene Therapy Market
Accelerates Growth in the AAV Gene Therapy Market The global
gene therapy market size is projected to
surpass USD 55.43 Billion by 2034 with a healthy CAGR of 19.60% from 2025 to
2034, as the parent ecosystem, establishes the
scientific, regulatory, and commercial foundation that directly accelerates the
rise of the AAV gene therapy market. As gene therapy gains broader acceptance in
clinical practice—especially in treating rare diseases, oncology, and inherited
disorders—AAV vectors benefit from strengthened R&D pipelines, improved
regulatory pathways, and a rapidly expanding manufacturing infrastructure. 🔹 The
parent gene therapy market drives technological innovation, including advanced
viral vector engineering, scalable bioprocessing systems, and improved delivery
mechanisms, all of which enhance AAV efficiency and safety. Together, these dynamics show how the broader
gene therapy landscape acts as both a catalyst and growth engine
for the AAV segment, enabling faster commercialization, wider therapeutic
applicability, and sustained market expansion. The
Complete Study is Now Available for Immediate Access | Download the Sample
Pages of this Report@ https://www.precedenceresearch.com/sample/1462 Key Ways the
Gene Therapy Market Supports AAV Gene Therapy Advancement 🔹 Shared
Clinical & Manufacturing Infrastructure – GMP suites, QC systems, and clinical trial networks built for
gene therapy reduce barriers for AAV development. Browse Detailed Insight 👉 https://www.precedenceresearch.com/gene-therapy-market Strategic Market Comparison: Positioning AAV
Gene Therapy Within the Global Gene Therapy Ecosystem
Parameter AAV
Gene Therapy Market Gene
Therapy Market (Parent Market) Market
Size in 2025 USD
2.85 Billion USD
11.07 Billion Market
Size in 2026 USD
3.74 Billion USD
13.24 Billion Market
Size by 2034 USD
23.54 Billion USD
55.43 Billion Growth
Rate (CAGR) 26.43%
(2025–2034) ~ 19.60 % (2025–2034) Market
Nature Specialized
sub-segment focused on AAV vector delivery Broad
market covering all gene therapy modalities Key
Drivers AAV9
adoption, rare disease focus, CNS & muscular disorder pipelines, FDA
approvals Oncology
dominance, rare disease demand, improved vector engineering, global
regulatory support Core
Technologies AAV
serotypes (AAV1–AAV12), engineered/synthetic capsids, IV/IT delivery AAV,
lentiviral, adenoviral, retroviral vectors, CRISPR & gene editing Dominating
Region North
America (42.19%) North
America (54%) Fastest-Growing
Region Asia
Pacific Asia
Pacific Major
Applications CNS,
muscular, ophthalmology, metabolic, hematology Oncology,
rare diseases, chronic disorders, regenerative medicine Industry
Maturity Early–growth
stage with rapid clinical expansion More
mature, with multiple approved therapies globally Growth
Catalysts from Parent Market Regulatory
familiarity, shared GMP capacity, strong investment pipeline Clinical
validation, robust trial ecosystem, major pharma involvement
Set up a meeting at your convenience
to get more insights instantly! https://www.precedenceresearch.com/schedule-meeting Case Study: AAV9 Gene Therapy Transforms
Early SMA Management Through a Precision-Driven Clinical Pathway A leading North American pediatric
neuromuscular center successfully redefined clinical outcomes for infants with Type
1 Spinal Muscular Atrophy (SMA) by implementing a structured,
multidisciplinary AAV9 gene therapy program. This shift—from reactive, crisis-focused
care to precision-timed intervention—demonstrates the transformative potential
of AAV-based therapies when embedded into an optimized care ecosystem. Challenge: Intervention: This model reduced variability, accelerated
treatment timelines, and ensured clinical consistency across all patient
cohorts. Outcomes: Early administration emerged as the defining
variable—infants treated before six months consistently demonstrated the
strongest functional recovery. Implications for the AAV Gene Therapy
Market: AAV Gene Therapy Market Key Regional
Analysis: North America Industry Analysis North America dominated the AAV gene therapy market in
2024, supported by its advanced research infrastructure, strong clinical trial
ecosystem, and substantial public and private investment in AAV technologies.
The region benefits from a high concentration of specialized hospitals,
well-established biotechnology hubs, and regulatory frameworks that encourage
rapid development and approval of advanced therapies. The United States leads the market with extensive
manufacturing capacity, FDA-backed innovation programs, and numerous
academic–industry collaborations, while Canada continues to grow through
national genomic
medicine initiatives, expanded rare disease research funding, and rising
partnerships with global biotech companies. These factors collectively
reinforce North America’s position as the primary center for AAV gene therapy
innovation and commercialization. Asia Pacific Industry Trends Asia Pacific is expected to experience significant
expansion in the AAV gene therapy market over the forecast period, driven by
rising investment in genetic medicine, rapid biomanufacturing advancements, and
government-supported rare disease programs. China is leading regional growth by
constructing new GMP-certified AAV production facilities and increasing
clinical trial activity, while Japan supports innovation through accelerated
regulatory pathways and strong academic, industry collaboration. South Korea is emerging as a competitive hub with
dedicated cell
and gene therapy industrial clusters and national biotech
development strategies, and India is gradually expanding its capabilities
through public–private partnerships and improved manufacturing infrastructure.
Together, these developments position Asia Pacific as one of the
fastest-growing regions for AAV gene therapy research, production, and clinical
adoption. ✚ Related
Topics You May Find Useful: ➡️ Adeno-Associated Virus Vector Manufacturing
Market:
Explore how scalable vector production is becoming the backbone of
next-generation gene therapy expansion ➡️ Single Dose Gene Therapy Market:
Understand why one-time curative treatments are reshaping pricing models,
clinical workflows, and patient outcomes ➡️ Cell and Gene Therapy Quality Control &
Analytics Market:
See how advanced QC frameworks and analytical tools are ensuring safety, purity,
and regulatory compliance in complex therapies ➡️ Viral Vectors-Based Gene Therapy for
Non-Human Primates Market:
Analyze how NHP-focused vector research is accelerating translational insights
for human gene therapy programs ➡️ Cell and Gene Therapy Infrastructure and
Delivery Models Market:
Discover how hospitals are redesigning infrastructure, infusion suites, and
operational models for gene therapy readiness ➡️ Neurological Rare Disease Biologics Market:
Track breakthroughs in biologics targeting ultra-rare CNS disorders with high
unmet need ➡️ Cell and Gene Therapy CDMO Market:
Examine how CDMOs are scaling manufacturing capacity, innovation, and
regulatory support for global gene therapy pipelines ➡️ Next-Gen Delivery Systems for Genetic Drug
Market:
Learn how novel delivery platforms are improving tissue targeting, durability,
and safety in genetic medicine ➡️ Cancer Gene Therapy Market:
Explore how genetic medicines are transforming oncology through targeted tumor
modulation and personalized treatments ➡️ RNA Editing Therapies Market:
Gain insights into precision RNA-level interventions driving the next frontier
of functional genetic repair ➡️ Gene
Silencing Market:
Understand how gene-suppression technologies are enabling durable therapeutic
effects in previously untreatable diseases AAV Gene Therapy Market Segmentation
Analysis: Therapeutic Area Analysis: The neurological disorders segment accounted for the
largest share of 29.4% in the AAV gene therapy market in 2024, reflecting the
strong demand for advanced treatments targeting complex neurodegenerative and
genetic conditions. This dominance is supported by the rapid rise in diseases
such as Alzheimer’s, Parkinson’s, spinal muscular atrophy, and rare inherited
neuropathies, where conventional treatments offer limited effectiveness. The muscular disorders segment is projected to grow at
the fastest CAGR from 2025 to 2034, driven by rising awareness of genetic
muscle diseases, advancements in genome delivery methods, and the growing
pipeline of AAV-based therapies for conditions such as Duchenne muscular
dystrophy and limb-girdle muscular dystrophy. Pharmaceutical and biotech
companies are heavily investing in next-generation vector engineering and
optimized dosing strategies to improve treatment durability and safety. Growing
collaboration between research institutions, increased patient advocacy
programs, and supportive regulatory pathways are further accelerating the
development and market expansion of muscular disorder therapies. Vector Serotype Analysis: In 2024, the AAV9 serotype contributed the largest market
share at 27.60%, solidifying its role as one of the most widely used vectors in
gene therapy. AAV9’s ability to efficiently target both systemic tissues and
the central nervous system makes it ideal for treating a broad range of genetic
diseases. Its extensive use in approved therapies and late-stage clinical
trials has contributed to its strong market presence. Continued advancements in
AAV9 manufacturing and growing adoption in pipeline programs across neurology,
cardiology, and metabolic disorder treatments further reinforce its market
leadership. Engineered, synthetic, and hybrid AAV capsids are
expected to witness notable growth during the forecast period as developers
seek improved vector specificity, enhanced tissue tropism, and reduced immune
responses. These next-generation capsids are designed to overcome limitations
associated with naturally occurring AAV types, enabling more effective gene
delivery with lower doses. Growing R&D investment in precision gene
therapy, combined with the increasing shift toward customized vectors for rare
and ultra-rare diseases, is driving strong interest and adoption in this
rapidly evolving segment. Route of Administration Analysis: The intravenous (I.V.) route of administration captured
36.80% of the market share in 2024, making it the most widely used delivery
method for AAV-based treatments. Its non-invasive nature, ease of
administration, and ability to distribute vectors throughout the bloodstream
make it suitable for treating multi-organ diseases and systemic genetic
disorders. Manufacturers and clinical researchers prefer I.V. delivery due to
its well-established safety profile and broad applicability across pediatric
and adult patient populations. Ongoing improvements in dosing regimens and
vector engineering are further supporting the strong use of intravenous
administration in clinical practice. The intrathecal (I.T.) route is projected to expand at a
notable CAGR over the forecast period, driven by increasing use of targeted CNS
delivery for neurological disorders. Intrathecal injection enables direct
deposition of AAV vectors into the cerebrospinal fluid, allowing highly
efficient gene transfer to spinal and brain tissues while minimizing systemic
exposure. This route is gaining momentum as more therapies for ALS, spinal
muscular atrophy, and other CNS disorders progress through clinical stages. Its
rising adoption is also supported by ongoing advancements in minimally invasive
neurosurgical delivery techniques. Application Stage Analysis: Clinical therapies held the largest market share of 48.7%
in 2024, reflecting the rapid expansion of AAV gene therapy candidates
advancing through Phase I–III clinical trials. A growing number of
biopharmaceutical companies are increasing their R&D investments to
accelerate the development of novel treatments targeting rare genetic diseases
and chronic disorders. Strong support from regulatory agencies, coupled with expanding
patient enrollment and improved clinical trial infrastructure, has further
strengthened the position of this segment. These factors collectively highlight
the robust progress in translating AAV-based innovations from laboratory
research to human studies. The commercialized therapies segment is expected to grow
significantly over the forecast period, supported by increasing regulatory
approvals and broader market acceptance of AAV gene therapies. As more
therapies demonstrate strong safety and long-term efficacy data, adoption is
rising across global healthcare systems. Growing payer support, expanded
reimbursement programs, and improvements in vector production scalability are
all contributing to the rapidly evolving commercial landscape. The segment’s
growth will also benefit from expanding indications and ongoing progress in
addressing manufacturing bottlenecks. Manufacturing Type Analysis: In-house manufacturing accounted for the largest share,
54.1%, in 2024, as companies sought greater control over production quality,
timelines, and regulatory compliance. Building internal AAV production capacity
allows biopharma companies to streamline scale-up processes and maintain
consistency in vector purity and potency. The complexity of AAV manufacturing,
combined with increasing regulatory scrutiny, has motivated developers to
invest heavily in their own GMP-compliant facilities. This trend further
supports rapid clinical development and minimizes dependency on external
suppliers. Contract development and manufacturing organizations
(CDMOs) and dedicated vector production facilities are anticipated to expand at
a notable CAGR, driven by increasing outsourcing demand from small- and
mid-sized biotech companies. As AAV therapies progress toward commercialization,
the need for scalable, high-quality manufacturing has intensified. CDMOs
provide specialized expertise, advanced bioprocessing technologies, and
large-scale capacity, making them essential partners in accelerating product
development. The surge in global gene therapy pipelines is expected to further
boost demand for third-party manufacturing services. End User Analysis: The pharmaceutical and biotechnology companies segment
generated the largest market share at 52.8% in 2024 due to significant R&D
investments, strategic partnerships, and ongoing efforts to develop innovative
AAV-based therapies. These companies are leading the charge in clinical trial
activity, manufacturing expansion, and commercialization strategies. Their
strong financial capability and access to advanced scientific resources
position them at the forefront of the AAV gene therapy landscape. Continuous
collaboration with academic institutions and technology providers further
strengthens this segment’s market dominance. Contract research organizations (CROs) are projected to
grow at a notable CAGR over the forecast period, as biopharmaceutical companies
increasingly outsource preclinical research, clinical trial management,
regulatory support, and analytical testing. The complexity of AAV gene therapy
development often requires specialized expertise and advanced laboratory
capabilities that CROs are well-equipped to provide. Rising global trial
activity, expanding regulatory requirements, and the need for cost-effective
development strategies are further propelling the expansion of CRO services
within the market. For questions or customization requests, please reach out
to us @ sales@precedenceresearch.com
| +1 804 441 9344 AAV Gene Therapy Market Top Companies ➢
Advanced
Energy Industries, Inc. ➢ MKS
Instruments, Inc. ➢ Samco
Inc. ➢ PVA
TePla AG ➢ Axcelis
Technologies, Inc. ➢ Lam
Research Corporation ➢ ULVAC,
Inc. ➢ Plasma
Etch, Inc. ➢ PIE
Scientific LLC ➢ Tokyo
Electron Limited (TEL) ➢ Trion
Technology, Inc. ➢ Diener
Electronic GmbH and Co. KG ➢ Nordson
MARCH ➢ Veeco
Instruments Inc. What is Going Around the Globe? 🔸In April 2025, the
U.S. Pharmacopeia (USP) launched a package of reference standards, materials,
and other resources to provide a clear understanding for developers and
manufacturers of AAV-based gene therapies. Fouad Atouf, Ph.D., Senior Vice
President of Global Biologics for USP, stated, “Innovative AAV-based therapies
are approved and in development for many therapeutic categories, including
previously untreatable genetic and rare diseases.” (Source: https://www.usp.org) AAV Gene Therapy Market Segmentation: 🔹 Neurological
Disorders → Spinal Muscular Atrophy (SMA) (e.g., Zolgensma) → Parkinson's Disease → Alzheimer's Disease → Rett Syndrome → Amyotrophic Lateral Sclerosis (ALS) → Canavan Disease → Aromatic L-amino acid decarboxylase (AADC) Deficiency → Huntington's Disease → Friedreich's Ataxia 🔹 Ophthalmic
Disorders → Leber Congenital Amaurosis (LCA) (e.g., Luxturna) → Retinitis Pigmentosa → Wet Age-related Macular Degeneration (AMD) → Stargardt Disease → Choroideremia 🔹 Hematologic
Disorders → Hemophilia A (e.g., Roctavian) → Hemophilia B (e.g., Hemgenix) → Thalassemia → Sickle Cell Disease 🔹 Muscular
Disorders → Duchenne Muscular Dystrophy (DMD) (e.g., Elevidys) → Limb-Girdle Muscular Dystrophy (LGMD) 🔹 Metabolic
Disorders → Alpha-1 Antitrypsin Deficiency → Phenylketonuria (PKU) → Glycogen Storage Diseases → Hereditary Lipoprotein Lipase Deficiency (LPLD) (e.g.,
Glybera - though withdrawn) 🔹 Rare
Genetic Disorders (Other) → Mucopolysaccharidosis (MPS) → Batten Disease → Cystic Fibrosis → Sanfilippo Syndrome 🔹 Oncology/Cancer → Various cancer types where AAVs are used for targeted
delivery of anti-cancer genes. → Cardiovascular Diseases By Vector Serotype 🔹 AAV1 🔹 AAV2 🔹 AAV3 🔹 AAV4 🔹 AAV5 🔹 AAV6 🔹 AAV7 🔹 AAV8 🔹 AAV9 🔹 AAV10 🔹 AAV11 🔹 AAV12 🔹 Engineered/Synthetic/Hybrid
Capsids By Route of Administration 🔹 Intravenous
(I.V.) 🔹 Intrathecal
(I.T.) (for CNS delivery) 🔹 Intraocular
(I.O.) (e.g., intravitreal, subretinal) 🔹 Intramuscular
(I.M.) 🔹 Intracerebral 🔹 Subcutaneous 🔹 Local/Direct
Injection (e.g., into specific organs) By Application Stage 🔹Preclinical
Therapies 🔹Clinical
Therapies → Phase I → Phase II → Phase III 🔹Commercialized
Therapies By Manufacturing Type 🔹In-house
Manufacturing 🔹CDMOs/Vector
Production Facilities By End-User 🔹Pharmaceutical
and Biotechnology Companies 🔹Academic
and Research Institutes 🔹Contract
Research Organizations (CROs) By Region 🔹North
America 🔹Europe 🔹Asia-Pacific 🔹Latin
America 🔹Middle
East and Africa Thanks for reading
you can also get individual chapter-wise sections or region-wise report
versions such as North America, Europe, or Asia Pacific. Don’t Miss Out!
| Instant Access to This Exclusive Report 👉 https://www.precedenceresearch.com/checkout/6873 You can place an order or ask any questions,
please feel free to contact at sales@precedenceresearch.com | +1 804 441 9344 Stay Ahead with Precedence
Research Subscriptions Unlock exclusive access to powerful market intelligence,
real-time data, and forward-looking insights, tailored to your business. From
trend tracking to competitive analysis, our subscription plans keep you
informed, agile, and ahead of the curve. Browse Our Subscription Plans@ https://www.precedenceresearch.com/get-a-subscription About Us Precedence Research is a global market intelligence and
consulting powerhouse, dedicated to unlocking deep strategic insights that
drive innovation and transformation. With a laser focus on the dynamic world of life sciences, we
specialize in decoding the complexities of cell and gene therapy,
drug development, and oncology markets, helping our clients stay
ahead in some of the most cutting-edge and high-stakes domains in healthcare.
Our expertise spans across the biotech and pharmaceutical ecosystem, serving
innovators, investors, and institutions that are redefining what’s possible in regenerative medicine, cancer care, precision
therapeutics, and beyond. Web: https://www.precedenceresearch.com ✚ Explore
More Market Intelligence from Precedence Research: ➡️
Digital Therapeutics:
How software-based interventions are restructuring chronic-disease management
and clinical-grade behavioral therapy ➡️
Life Sciences Growth:
Forces driving expansion across biotech, biopharma, and advanced therapeutic
platforms ➡️
Viral Vector Gene Therapy Manufacturing:
Manufacturing constraints, scalability limits, and innovations shaping next-generation
gene-delivery systems ➡️
Wellness Transformation:
How prevention-centric health models are shifting consumer behavior, product
pipelines, and care delivery ➡️
Generative AI in Healthcare:
How generative models are unlocking new diagnostics, clinical automation, and
patient-care innovations Our Trusted Data Partners: Towards Healthcare | Nova One
Advisor | Onco Quant | Statifacts Get Recent News 👉 https://www.precedenceresearch.com/news For Latest Update Follow Us: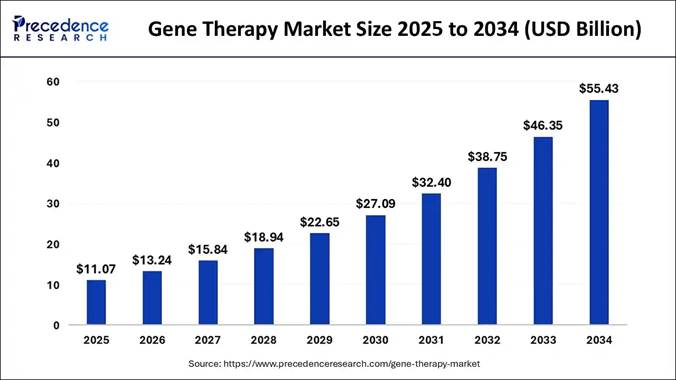
🔹 As approvals for
gene therapies increase globally, regulatory agencies become more familiar with
AAV-based products, reducing uncertainty and accelerating development timelines
for AAV pipelines.
🔹 Greater industry
investment—from pharma, biotech, and venture capital—flows into specialized AAV
vector programs, supported by the larger
trend toward personalized medicine and
genetic medicine.
🔹 Expansion of High-Value Therapeutic Areas – CNS, muscular,
metabolic, and rare genetic programs rely heavily on AAV vectors, boosting
downstream demand.
🔹 Technology Spillover from the Parent Market – Breakthroughs
in viral vector engineering, computational optimization, and immune-evasion
strategies strengthen AAV pipelines.
🔹 Investment Momentum & Regulatory Tailwinds – Growth in
the gene therapy market attracts capital, accelerates late-stage AAV trials,
and strengthens regulatory alignment.

Before gene therapy integration, infants with Type 1 SMA often arrived late in
the disease course, faced rapid motor deterioration, and required prolonged
ventilatory support. Standard treatments slowed decline but could not alter the
underlying genetic pathology. The center needed a model that enabled ultra-early
intervention and improved long-term outcomes.
The hospital created a dedicated AAV9 gene therapy pathway that included:
The program delivered significant clinical and operational improvements:
This case underscores why neurological disorders dominate AAV adoption
and why AAV9 remains the leading vector in clinical pipelines. It highlights
the importance of:
For inquiries regarding discounts, bulk purchases, or customization requests,
please contact us at sales@precedenceresearch.com
AAV vectors particularly AAV9, have demonstrated strong therapeutic potential
due to their capability to cross the blood–brain barrier, making them a
preferred choice for CNS-targeted therapies. Increasing funding for
neurological research, combined with expanding clinical trials targeting the
central nervous system, continues to strengthen this segment’s position.
By
Therapeutic Area/Disease Indication




