News
After a patient safety signal and then death, the FDA in October 2025 placed holds on two of the company’s CRISPR programs for hereditary transthyretin amyloidosis.
FEATURED STORIES
Some 200 rare disease therapies are at risk of losing eligibility for a pediatric priority review voucher, a recent analysis by the Rare Disease Company Coalition shows. That could mean $4 billion in missed revenue for already cash-strapped biotechs.
Together with robust data-driven modeling, rethinking regulation and data use could push forward a notoriously challenging field.
From opening new therapeutic mechanisms to repairing neuronal damage, investigational molecules from Ventyx Therapeutics, AC Immune, Gain Therapeutics and more could shape the future of Parkinson’s disease treatment.
FROM OUR EDITORS
Read our takes on the biggest stories happening in the industry.
The FDA’s rare pediatric disease priority review voucher program missed reauthorization at the last minute in 2024; advocates have been fighting to get it back ever since.
THE LATEST
The FDA on Monday only granted Liquidia’s Yutrepia tentative approval, keeping it off the U.S. market until after rival United Therapeutics’ exclusivity expires in May 2025.
The Danish company on Saturday announced plans to increase production of its vaccine Jynneos on the heels of the World Health Organization last week declaring mpox a global health emergency.
SIGA Technologies’ TPOXX did not outperform placebo at resolving lesions in patients with clade I mpox, the new strain that has spread through parts of Africa and is reaching beyond the continent.
The unit’s closure comes as Genentech’s parent Roche rethinks its cancer business, an effort that has included the discontinuation of three early-stage candidates and a T-cell partnership with Adaptimmune.
By building and nurturing a strong personal brand, you can benefit in multiple ways, including enhancing your credibility, attracting opportunities and inspiring investor confidence.
Psychedelic drug developers are undeterred by the FDA’s Complete Response Letter for the company’s MDMA therapy for PTSD, and experts expect Lykos will ultimately obtain approval.
Regeneron, Akouos and Mass Eye and Ear are testing therapies that can reverse genetic protein deficiency to restore hearing, with promising early results.
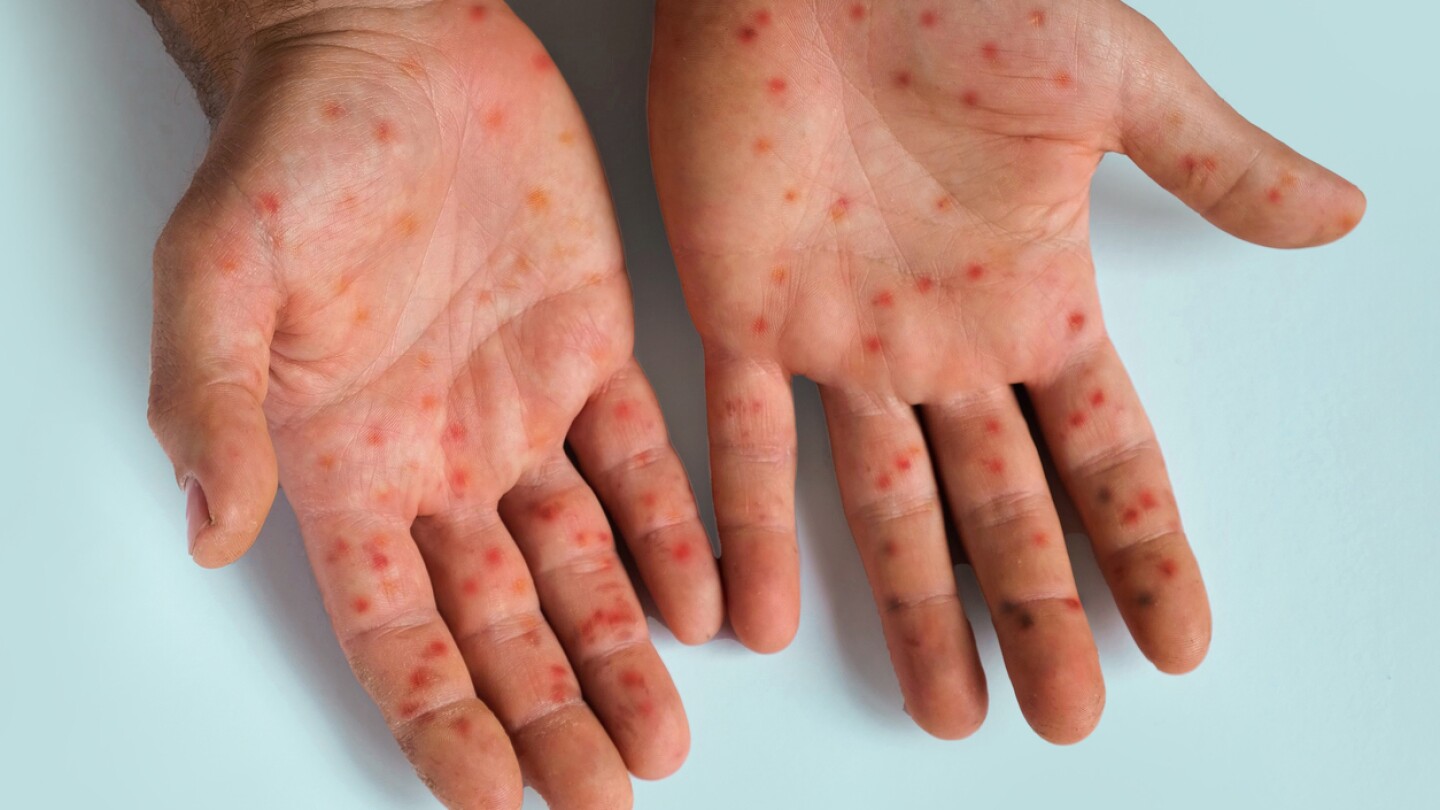
The entry of new players and new approaches into the ATTR-CM space could help bring down the cost of treatment, experts say.
For the Biden-Harris administration to compare the newly announced negotiated Medicare prices to the list prices for these drugs is, at best, not very meaningful. At worst, it’s disingenuous.
Lykos will lay off approximately three-quarters of its staff amidst a reorganization aimed at helping the company complete a regulatory resubmission for its MDMA-assisted therapy.