The 2022 American Academy of Dermatology Annual Meeting saw several milestones for treatment explorations for inflammatory diseases. Here is some of the major news:
The 2022 American Academy of Dermatology Annual Meeting saw several milestones in treatment explorations for inflammatory diseases. Here’s some of the major news shared at the event.
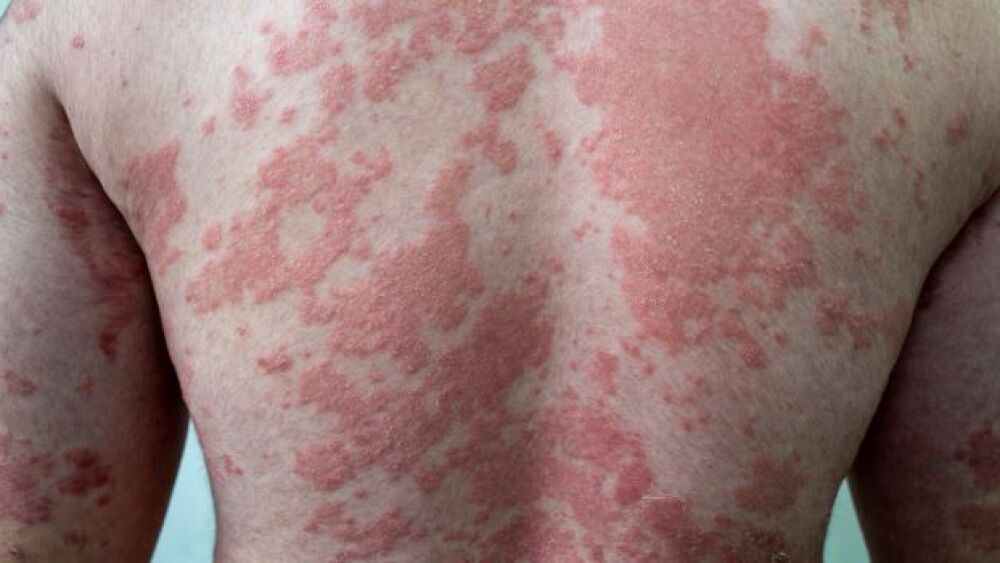
Eli Lilly’s Dermatitis Drug Delivers
Eli Lilly and Company announced that its candidate monotherapy for dermatitis succeeded in delivering clinically significant outcomes.
Based on Eczema Area Severity, the ADvocate 1 and ADvocate 2 trials and Phase III programs ended with over 50% of moderate-to-severe dermatitis patients experiencing a drop in disease severity by at least 75% after 16 weeks with lebrikizumab. Lebrikizumab is an investigational, monoclonal antibody that binds IL-13 with high affinity to prevent IL-13Rα1/IL-4Rα heterodimer complex formation and subsequent signaling, thus inhibiting IL-13 effects more efficiently. IL-13 is a central mediator of atopic dermatitis, causing inflammations that drive itching, skin infection and thickening, and other skin barrier dysfunctions.
“Lebrikizumab is a novel treatment targeting the IL-13 pathway, which is the main cytokine driver of inflammation that is involved in AD. I’m encouraged by today’s data showing rapid improvements in skin, itch and quality-of-life measures,” commented Emma Guttman-Yassky, MD, Ph.D., the system chair of dermatology at the Icahn School of Medicine at Mount Sinai in New York and senior author of the ADvocate trial, in a statement.
ADcovate 1 and ADvocate 2 are ongoing 52-week randomized, double-blind, placebo-controlled, parallel-group Phase III trials evaluating the effect of lebrikizumab in adults and adolescents ages 12 years and up, weighing at least 40 kgs and living with moderate to severe atopic dermatitis. The primary endpoints were assessed using an Investigator Global Assessment (IGA) score and EASI-75. EASI-75 measures the severity and extent of the disease. Key secondary endpoints were measured using IGA, EASI, the Pruritis Numeric Rating Scale, the Sleep-Loss due to Pruritus Index and the Dermatology Life Quality Index.
Lebrikizumab received Fast Track designation from the U.S. Food and Drug Administration in 2019. The company has exclusive rights to develop and commercialize the drug in the U.S. and the rest of the world except Europe, where partner Almirall holds the exclusive rights.
Krystal’s B-VEC Shows Promise In Epidermolysis Bullosa
Krystal Biotech shared more detailed data from its ongoing Phase III GEM3 study of beremagene geperpavec (B-VEC) for treating dystrophic epidermolysis bullosa.
In the 2022 American Academy of Dermatology Annual Meeting presentation, primary investigator Peter Marinkovich, M.D. said that the drug met primary and secondary efficacy endpoints of complete wound healing compared to a placebo. 31 participants were observed at three- and six-month intervals. The drug is currently being investigated for its long-term efficacy in an open-label extension study.
“Following the initial report of the topline results, the latest data lend further strong support for our belief that B-VEC could correct this devastating disease at the molecular level and fulfill our founding mission to bring a convenient, non-invasive treatment to EB patients and their families,” commented Suma Krishnan, president of research and development at Krystal, in a press release.
Evelo’s Oral Psoriasis Drug Demonstrates Efficacy
Evelo Biosciences’ investigational oral treatment for psoriasis demonstrated significant safety and efficacy numbers from its initial Phase II trial, building optimism that the drug could also address many unmet needs across various stages of the disease.
EDP1815 is Evelo’s orally-delivered, anti-inflammatory, gut-restricted commensal microbe treatment for mild and moderate plaque psoriasis.
Participants were given either the drug or a placebo for 16 weeks and then followed up for up to 24 weeks. Researchers observed that EDP1815 did not lead to any safety issues or drug-related serious adverse events.
EDP1815 is one of Evelo’s three product candidates under development to address inflammatory diseases.
Nimbus Plaque Psoriasis Candidate Shows Positive Early Results
Nimbus Therapeutics shared that its candidate treatment for moderate to severe plaque psoriasis delivered promising outcomes from its Phase IB clinical trial that ended in April 2021.
NDI-034858, an investigational oral allosteric tyrosine kinase 2 inhibitor, was given to patient-participants over the course of 28 days in once-daily doses of 5 mg, 10 mg, 30 mg or a placebo.
At the end of the study period, researchers saw improvements across several measures, including the decreased thickness of lesional skin epidermis, decreased Ki67 expression, decreased expression of psoriaris-linked genes, up to 50% improvement in skin transcriptomes and resolution of elevated keratin-16 expression. Complete details are published in the study titled “Analysis of histologic, molecular and clinical improvement in moderate-to-severe psoriasis: Results from a Phase 1b trial of the novel allosteric TYK2 inhibitor NDI-034858.”