News
Following the FDA’s refusal to review Moderna’s investigational mRNA flu vaccine last week, Commissioner Marty Makary faced questions from the U.S. president about the agency’s handling of vaccines. It’s a clear signal that the tension long brewing at the drug regulator has now gone all the way to the top.
FEATURED STORIES
Many scientists-turned-CEOs paradoxically abandon scientific principles when it comes to commercializing their innovations. But applying the scientific method to business decisions can help life science entrepreneurs avoid common pitfalls, attract investment and ultimately bring transformative technologies to market.
FDA vouchers are normally a coveted prize for biopharma companies, but a surprise rejection for Disc Medicine’s rare disease drug has biopharma reconsidering.
PitchBook’s 2025 biopharma VC analysis clocked $33.8 billion in capital dispatched in 2025, mainly to companies with later-stage programs ready to roll into the clinic.
FROM OUR EDITORS
Read our takes on the biggest stories happening in the industry.
The FDA’s refusal to review Moderna’s mRNA-based flu vaccine is part of a larger communications crisis unfolding at the agency over the past nine months that has also ensnarled Sarepta, Capricor, uniQure and many more.
THE LATEST
Biohaven has suffered a few setbacks in recent months, including an FDA rejection and a missed $150 million benchmark payment, but CEO Vlad Coric looked for the brighter side at JPM, specifically emphasizing a serendipitous discovery that could get the company in the obesity game.
In November, Pfizer was reportedly looking to divest its stake in BioNTech, though the German biotech at the time denied these rumors.
The arrangement will boost AstraZeneca’s cell therapy portfolio as the pharma targets $80 billion in revenue by 2030.
The star of the acquisition, anti-IgE antibody ozureprubart, is being tested as a prophylactic treatment for food allergies, potentially setting up a competition for GSK with Roche’s Xolair.
The companies have an expansive clinical program for the mRNA neoantigen therapy intismeran autogene in combination with immuno-oncology heavyweight Keytruda.
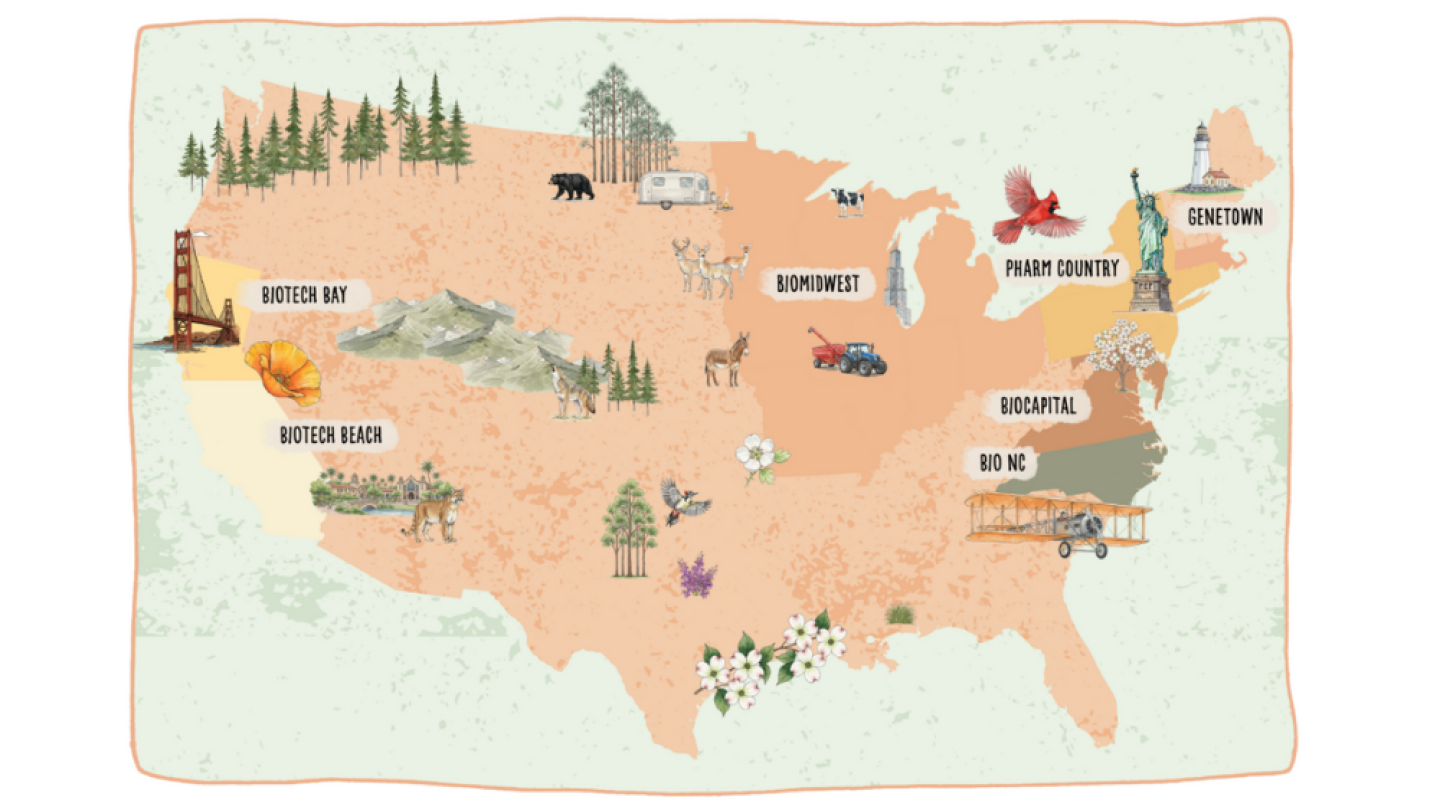
BioSpace has revealed its new 2026 Hotbed Maps, showcasing seven regional life sciences hot spots.
The initiative could tackle the first-mover disadvantage some CDMOs believe deters early customers, but leaders at companies including Novo Nordisk see hurdles to implementing the changes.
Henry Gosebruch, who has $3.5 billion in capital to deploy, is thinking broad as he steers the decades-old biotech out of years of turmoil.
In this bonus episode, BioSpace’s Vice President of Marketing Chantal Dresner and Careers Editor Angela Gabriel take a look at Q4 job market performance and what it signals for 2026.
Two more biopharma companies—the hair-growth specialist Veradermics and cancer-focused Eikon Therapeutics—have announced their IPOs this year. Meanwhile, Aktis Oncology began trading publicly earlier this month.