News
The company plans to divest a drug it has made for 40 years, citing increasing production costs and falling prices.
FEATURED STORIES
Corsera Health’s Chief Operating Officer Rena Denoncourt and CFO Meredith Kaya speak with BioSpace about the biotech’s mission and vision for the next generation of cardiovascular care.
Billions of dollars’ worth of cancer drugs are discarded each year. Manufacturers must refund Medicare for some of this waste. A data-driven approach offers a practical path to greater efficiency.
Sales of Merck’s longtime oncology blockbuster Keytruda will erode more starkly in about 2033 rather than 2029, predicts Bloomberg Intelligence, translating to some $22 billion more in revenue.
FROM OUR EDITORS
Read our takes on the biggest stories happening in the industry.
Following the FDA’s refusal to review Moderna’s investigational mRNA flu vaccine last week, Commissioner Marty Makary faced questions from the U.S. president about the agency’s handling of vaccines. It’s a clear signal that the tension long brewing at the drug regulator has now gone all the way to the top.
THE LATEST
At some point in your research career, you may find yourself transitioning from academia to industry or vice versa. To best set yourself up for success, adapt your approach to the specific scientific culture where you work.
As drug candidates discovered via AI move into later-stage clinical trials, the technology seems to be doing as promised: speeding drug development.
Biohaven has suffered a few setbacks in recent months, including an FDA rejection and a missed $150 million benchmark payment, but CEO Vlad Coric looked for the brighter side at JPM, specifically emphasizing a serendipitous discovery that could get the company in the obesity game.
In November, Pfizer was reportedly looking to divest its stake in BioNTech, though the German biotech at the time denied these rumors.
The arrangement will boost AstraZeneca’s cell therapy portfolio as the pharma targets $80 billion in revenue by 2030.
The star of the acquisition, anti-IgE antibody ozureprubart, is being tested as a prophylactic treatment for food allergies, potentially setting up a competition for GSK with Roche’s Xolair.
The companies have an expansive clinical program for the mRNA neoantigen therapy intismeran autogene in combination with immuno-oncology heavyweight Keytruda.
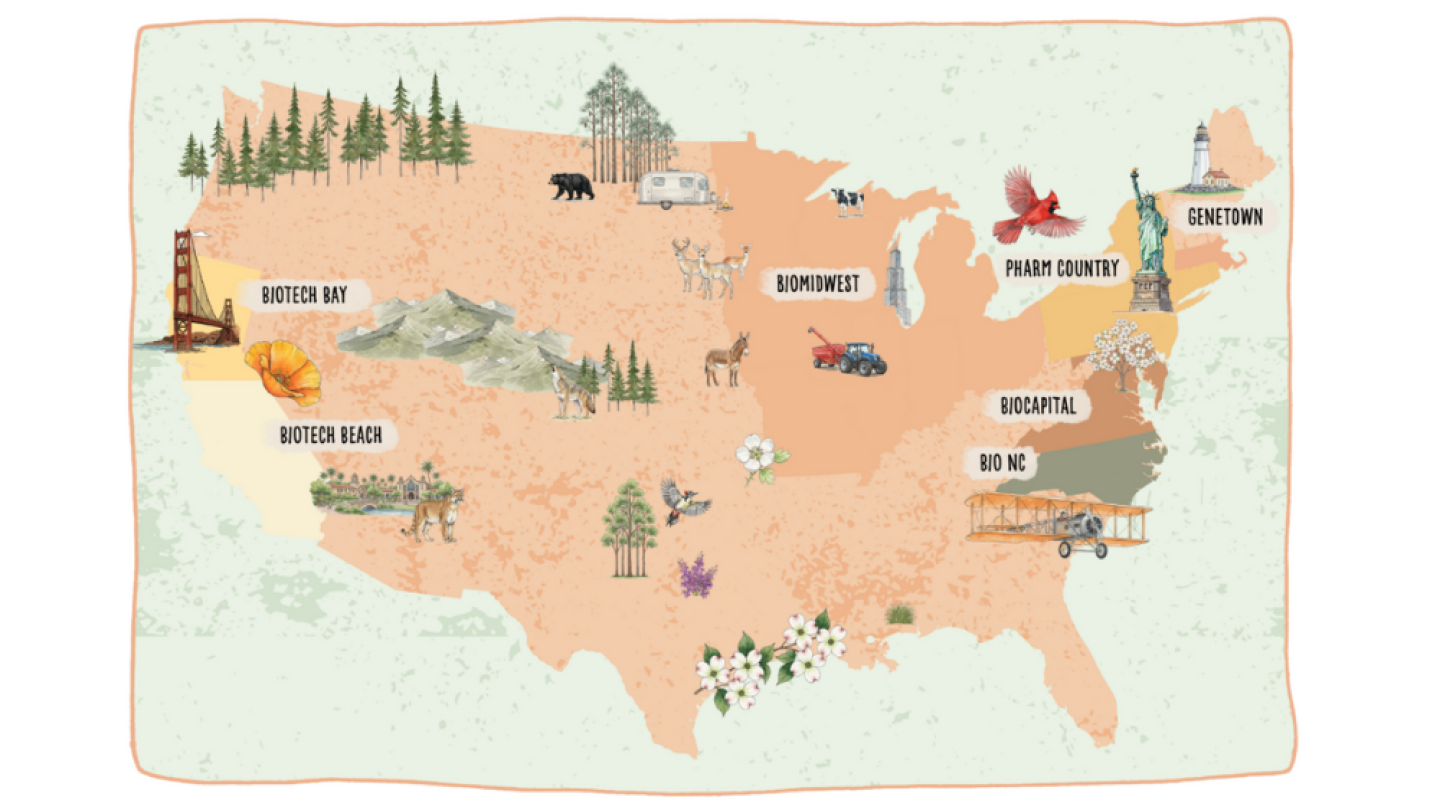
BioSpace has revealed its new 2026 Hotbed Maps, showcasing seven regional life sciences hot spots.
The initiative could tackle the first-mover disadvantage some CDMOs believe deters early customers, but leaders at companies including Novo Nordisk see hurdles to implementing the changes.
Henry Gosebruch, who has $3.5 billion in capital to deploy, is thinking broad as he steers the decades-old biotech out of years of turmoil.