News
Jay Bhattacharya will become the latest leader of the CDC on an acting basis, days after Jim O’Neill stepped aside.
FEATURED STORIES
PitchBook’s 2025 biopharma VC analysis clocked $33.8 billion in capital dispatched in 2025, mainly to companies with later-stage programs ready to roll into the clinic.
Long an R&D company that partnered off assets, RNAi biotech Ionis Pharmaceuticals shifted in 2025 to bring two medicines to market alone. Analysts are already impressed—and there’s more to come in 2026.
Regulatory uncertainty is no longer background noise. It is a material investment risk that reshapes how capital is deployed and pipelines are prioritized.
FROM OUR EDITORS
Read our takes on the biggest stories happening in the industry.
The FDA’s refusal to review Moderna’s mRNA-based flu vaccine is part of a larger communications crisis unfolding at the agency over the past nine months that has also ensnarled Sarepta, Capricor, uniQure and many more.
THE LATEST
The arrangement will boost AstraZeneca’s cell therapy portfolio as the pharma targets $80 billion in revenue by 2030.
The star of the acquisition, anti-IgE antibody ozureprubart, is being tested as a prophylactic treatment for food allergies, potentially setting up a competition for GSK with Roche’s Xolair.
The companies have an expansive clinical program for the mRNA neoantigen therapy intismeran autogene in combination with immuno-oncology heavyweight Keytruda.
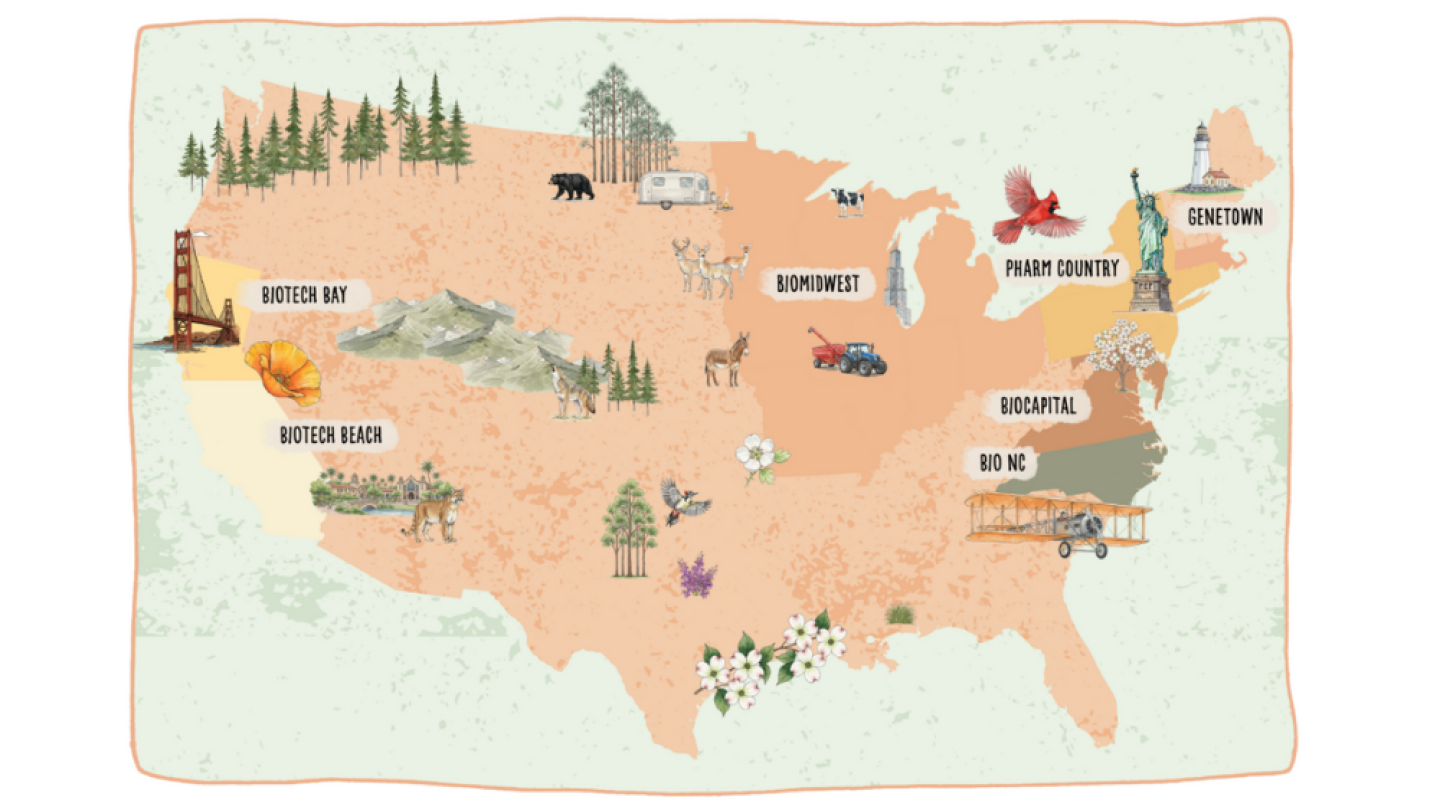
BioSpace has revealed its new 2026 Hotbed Maps, showcasing seven regional life sciences hot spots.
The initiative could tackle the first-mover disadvantage some CDMOs believe deters early customers, but leaders at companies including Novo Nordisk see hurdles to implementing the changes.
Henry Gosebruch, who has $3.5 billion in capital to deploy, is thinking broad as he steers the decades-old biotech out of years of turmoil.
In this bonus episode, BioSpace’s Vice President of Marketing Chantal Dresner and Careers Editor Angela Gabriel take a look at Q4 job market performance and what it signals for 2026.
Two more biopharma companies—the hair-growth specialist Veradermics and cancer-focused Eikon Therapeutics—have announced their IPOs this year. Meanwhile, Aktis Oncology began trading publicly earlier this month.
Despite the late-stage miss, analysts maintained confidence in the Epkinly program, with Truist Securities saying the result “doesn’t waver our optimism” regarding the bispecific antibody’s ongoing frontline trial.
Despite ushering in the current GLP-1 era, Novo Nordisk has fallen behind its chief rival Eli Lilly, which has exceeded the Danish pharma in terms of sales.