News
In this episode of Denatured, BioSpace’s head of insights Lori Ellis and Colin Zick, partner at Foley Hoag LLP, spend time discussing some of the points brought up in the Bioprocessing Summit last month. They explore the connections between hammers, AI, The Planet of the Apes and monoliths.
FEATURED STORIES
The FDA delivered two notable approvals for RSV immunization, UroGen overcame a negative advisory committee vote to secure an approval in bladder cancer, and more key regulatory nods from the past month.
In the race to make the most tolerable obesity drug, there seems to be no clear winner—at least not according to analysts parsing the data presented at the American Diabetes Association annual meeting this week.
Writing in JAMA, four former government officials warn that the Trump administration’s involvement in delaying the approval of Novavax’s COVID-19 vaccine could indicate a politicization of the drug approval processes that could ‘imperil public health.’
Job Trends
Regeneron Pharmaceuticals, Inc. (NASDAQ: REGN ) and Mammoth Biosciences, Inc., today announced a collaboration to research, develop and commercialize in vivo CRISPR-based gene editing therapies for multiple tissues and cell types.
FROM OUR EDITORS
Read our takes on the biggest stories happening in the industry.
Unpredictable communication and a lack of transparency are eroding the industry’s and the public’s trust. The FDA, experts agree, needs to take control of the narrative.
THE LATEST
In late May, a patient died after receiving Rocket Pharmaceuticals’ investigational gene therapy for Danon disease, spurring the hold. After lowering the dose and changing the regimen of immune modulators patients receive, the company has received FDA clearance for the trial to continue.
The FDA recommends that companies use overall survival as a primary endpoint for clinical trials where feasible. The new guidance follows the surprising return of CBER Head Vinay Prasad, who has previously argued for prioritizing OS.
With its structural changes, CSL expects to generate $500 to $550 million in annualized savings over the next three years.
Regulations aiming to lower the cost of vital medicines will instead end up restricting access and disincentivizing R&D.
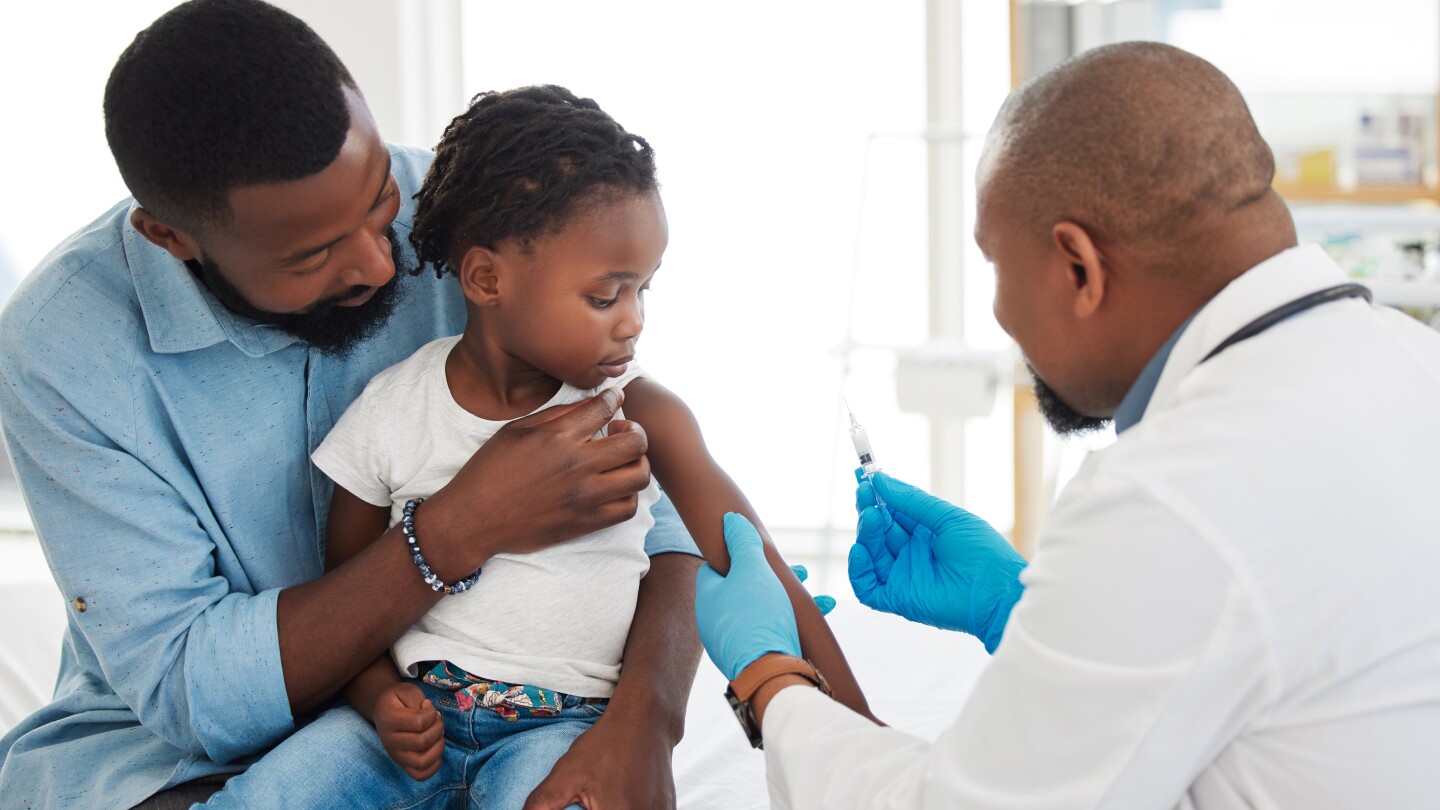
The CDC no longer recommends COVID-19 vaccines for healthy children and healthy pregnant women, a position that has been opposed by leading medical societies.
A draft copy of an upcoming MAHA report reveals a strategy in lockstep with recent HHS actions such as reviving the Task Force on Safer Childhood Vaccines; Viking Therapeutics reports robust efficacy from mid-stage oral obesity candidate but is tripped up by tolerability concerns; Novo Nordisk wins approval for Wegovy in MASH; and Lilly takes a pricing stand.
Media coverage can help biopharma executives connect with, inform and inspire the public. In this column, Kaye/Bassman’s Michael Pietrack and three communications experts share how to make the most of these opportunities.
Capricor Therapeutics met with the FDA last week for a type A meeting, during which CEO Linda Marbán aimed to explain to the regulator that it got it wrong. Capricor plans to resubmit the application based on deramiocel’s existing dataset.
The Trump administration’s ever-changing tariffs and Most Favored Nation drug pricing are part of a blizzard of unclear, potentially illegal tactics that leave observers throwing their hands in the air.
Drugs are being invented and manufactured right here in the U.S. by Americans, for Americans. So why doesn’t the industry hold the same respect as steelworkers or other all-American pursuits?