News
Vinay Prasad, the FDA’s Center for Biologics Evaluation and Research head, is accused of interpersonal impropriety as pushback builds against his decision to reject Moderna’s influenza vaccine candidate.
FEATURED STORIES
The rare disease drugmaker is facing potential competitors for achondroplasia drug Voxzogo. Is a big M&A deal with two approved assets enough to maintain investor interest?
The FDA issued a rare Refusal-to-File letter to Moderna over its mRNA-based influenza vaccine application, in an unusual move that sent the biotech’s shares tumbling.
A rapturous response to data published last year for Pelage’s hair loss candidate overwhelmed the biotech. Now, the company is ready to show the world the science behind the breakthrough.
FROM OUR EDITORS
Read our takes on the biggest stories happening in the industry.
Novo Nordisk and Eli Lilly have been battling head-to-head in an exploding obesity market. They should never have been compared apples to apples.
THE LATEST
With no evidence to support the claim that 10 children died due to COVID-19 vaccines, experts unpack the impact of CBER chief Vinay Prasad’s leaked vaccine memo.
While Imvax’s autologous immunotherapy IGV-001 missed the primary endpoint of progression-free survival in a Phase IIb trial, the company will request a meeting with the FDA to discuss next steps for “synergistic” treatment.
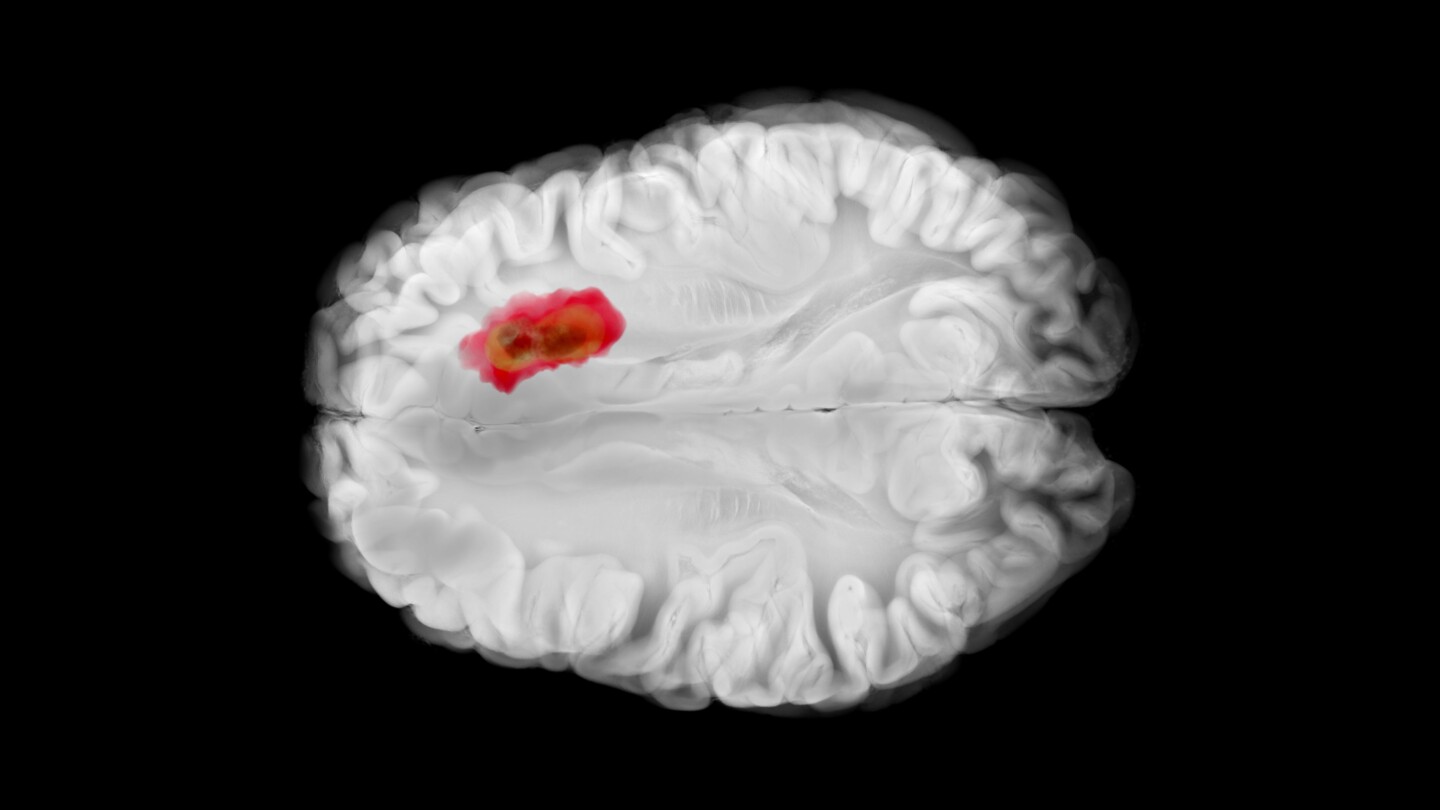
After covering the Alzheimer’s space through every high and low, BioSpace’s Annalee Armstrong welcomes back Roche for the 2026 Alzheimer’s Renaissance.
Trontinemab lowered amyloid levels below the threshold of positivity in 92% of treated patients.
This week’s meeting of the Advisory Committee on Immunization Practices will be led by Kirk Milhoan, a physician and pastor who recently claimed that COVID-19 vaccines contained a contamination that causes cancer.
Agentic AI can help FDA staff manage meetings, conduct pre-market reviews and validate reports, among other tasks, though the agency emphasized that using this technology is optional for its employees.
Following FDA rejections, Regeneron and Scholar Rock are turning to other facilities to clear regulatory logjams created by quality problems at an ex-Catalent facility in Indiana. Novo Nordisk, meanwhile, has been tight-lipped about whether its own FDA applications have been affected.
U.K.-based pharmas will not face tariffs as long as Donald Trump is president, according to the agreement.
Following Novo Nordisk’s price cuts for its own GLP-1 medicines, Eli Lilly is offering discounts for the obesity drug purchased through LillyDirect. Both pharmas recently struck a deal with the White House for cheaper prices via the yet-to-be-launched TrumpRx.
Protego Biopharma is advancing a small-molecule drug that helps light chain proteins fold correctly, in turn addressing the underlying biological cause of AL amyloidosis.