News
Disc Medicine’s leadership tried to express optimism that its rare disease therapy bitopertin can be approved based on a Phase 3 trial set to begin shortly. However, analysts are worried that the protocol was developed with former FDA leaders.
FEATURED STORIES
The FDA’s refusal to review Moderna’s mRNA-based flu vaccine is “part of a disturbing pattern” of moving regulatory goalposts, according to Clay Alspach, executive director of the Alliance for mRNA Medicines. Meanwhile, streamlined communications with regulators in other countries pave the way for rapid uptake of novel modalities.
The current state of political affairs in the U.S. does not bode well for the direction of that turn. The country is at real risk of losing its long-held lead in biotech innovation.
The FDA’s refusal to review Moderna’s mRNA-based flu vaccine is part of a larger communications crisis unfolding at the agency over the past nine months that has also ensnarled Sarepta, Capricor, uniQure and many more.
FROM OUR EDITORS
Read our takes on the biggest stories happening in the industry.
The FDA issued a rare Refusal-to-File letter to Moderna over its mRNA-based influenza vaccine application, in an unusual move that sent the biotech’s shares tumbling.
THE LATEST
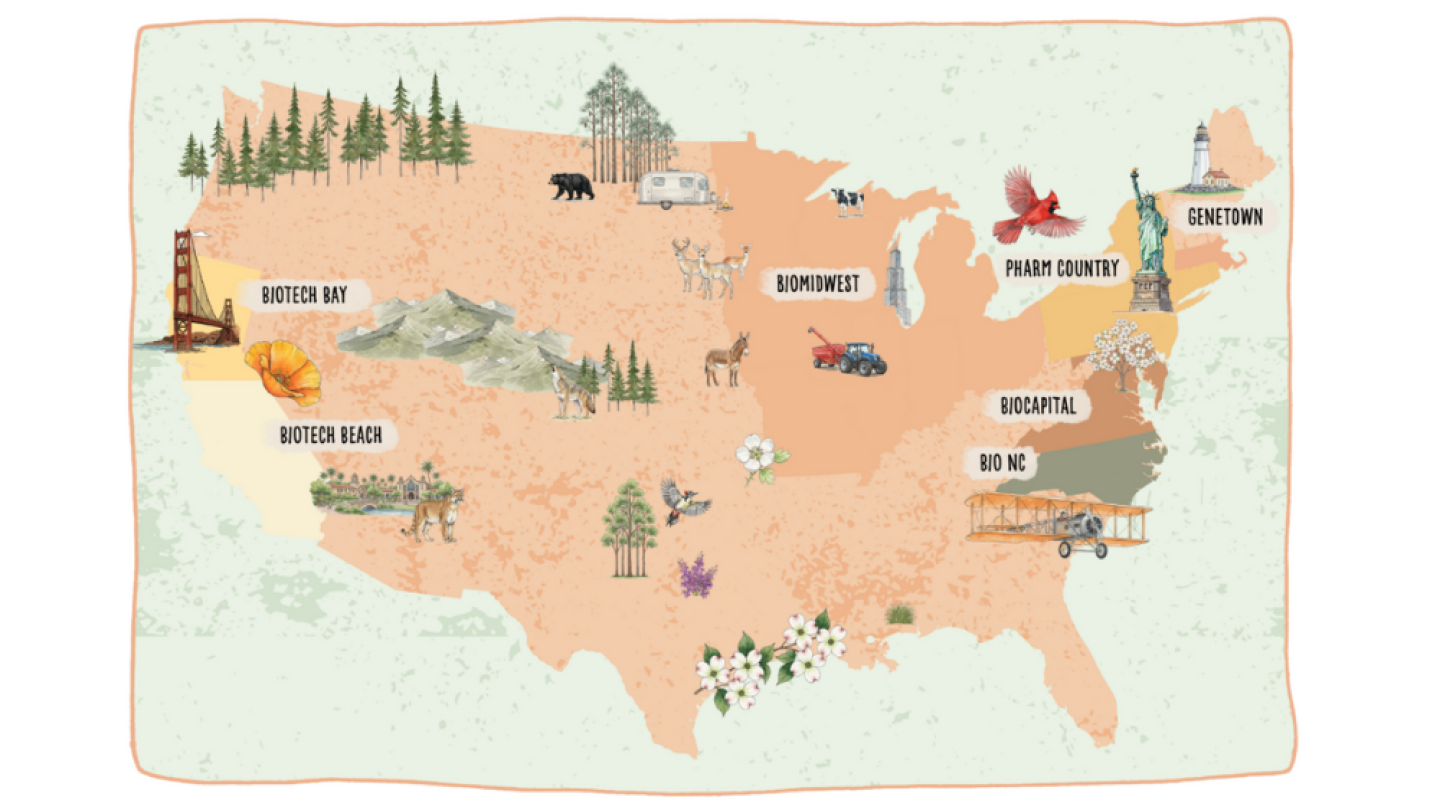
BioSpace has revealed its new 2026 Hotbed Maps, showcasing seven regional life sciences hot spots.
The initiative could tackle the first-mover disadvantage some CDMOs believe deters early customers, but leaders at companies including Novo Nordisk see hurdles to implementing the changes.
Henry Gosebruch, who has $3.5 billion in capital to deploy, is thinking broad as he steers the decades-old biotech out of years of turmoil.
In this bonus episode, BioSpace’s Vice President of Marketing Chantal Dresner and Careers Editor Angela Gabriel take a look at Q4 job market performance and what it signals for 2026.
Two more biopharma companies—the hair-growth specialist Veradermics and cancer-focused Eikon Therapeutics—have announced their IPOs this year. Meanwhile, Aktis Oncology began trading publicly earlier this month.
Despite the late-stage miss, analysts maintained confidence in the Epkinly program, with Truist Securities saying the result “doesn’t waver our optimism” regarding the bispecific antibody’s ongoing frontline trial.
Despite ushering in the current GLP-1 era, Novo Nordisk has fallen behind its chief rival Eli Lilly, which has exceeded the Danish pharma in terms of sales.
With inspections always on the table, LOTTE Biologics is pushing a proactive, quality first culture across its dual hubs in Syracuse and Songdo, backing its new single‑use ADC facility with rigorous training, self‑audits and real‑time regulatory surveillance.
BioSpace’s 2025 Q4 U.S. Life Sciences Job Market update highlights early signs of stabilization in biopharma hiring, with modest gains in job postings, slowing layoffs, and cautiously improving sentiment heading into 2026.
Following the hard-won success of early anti-amyloid drugs, a new generation of Alzheimer’s modalities—from tau-targeting gene silencers to blood-brain barrier delivery platforms—is entering the pipeline to anchor future combination therapies.