Third quarter fiscal 2020 revenue of approximately $221.6 million, an increase of 10% compared to revenue of $200.6 million for the same period of fiscal 2019.

SAN FRANCISCO--(BUSINESS WIRE)-- Abiomed, Inc. (NASDAQ: ABMD), a leading provider of breakthrough heart support technologies today reported preliminary, unaudited, third quarter fiscal 2020 revenue of approximately $221.6 million, an increase of 10% compared to revenue of $200.6 million for the same period of fiscal 2019.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20200113005332/en/
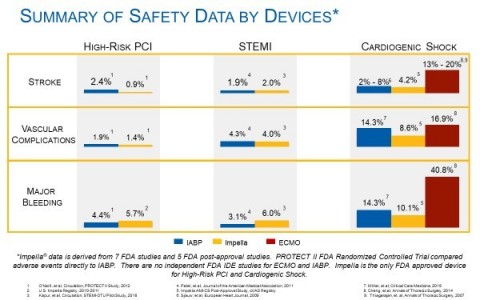
Summary of Safety Data by Devices (Graphic: Business Wire)
- Abiomed had a strong start to the quarter across all geographies, highlighted by 24% global revenue growth and 16% growth in U.S. patient usage in October.
- Preliminary unaudited total U.S. revenue grew 8% to $185.6 million from $172.6 million in the prior fiscal year. U.S. patient usage grew 5% in comparison with the same period of fiscal 2019.
- Outside the U.S., total revenue for the quarter totaled $36.0 million, an increase of 29% compared to revenue of $28.0 million during the same period of fiscal 2019, due to strength in Germany and Japan.
- In the quarter, the company was negatively impacted by two presentations at a conference on November 17, which the company believes are based on misleading analyses. The company is taking the following actions:
- Abiomed’s Medical Office completed a review that determined the presentations are misleading based on older, non-FDA audited, observational data on less than 4% of our patients treated over a 10-year period. The response is detailed in our press release and video publication review issued on November 19. Abiomed has also initiated processes to increase the publications of real-world evidence for mechanical circulatory support in the NCDR database which will include patients that are escalated on an IABP and ECMO.
- Abiomed has selected physician experts in the field of circulatory support to create a dedicated faculty for education and training on PCI and hemodynamic support. These programs will include a national user meeting, educational programs with onsite, in-house and online training, physician proctoring, establishing heart recovery centers of excellence and a password protected online community for physicians.
- Initiating a multi-city road show in Q4 to review with physicians Impella’s current clinical data, FDA studies and best practices that support Impella’s indications for high-risk PCI, cardiogenic shock and right heart failure.
- Abiomed will also be working with appropriate societies and hospital systems to communicate the audited and published results from seven FDA studies, five on-going FDA post-approval studies, and independent physician-led initiates such as NCSI, the INOVA heart team approach, and The Shock Working Group. These studies demonstrate improved outcomes with our best practices for high-risk PCI, cardiogenic shock and right heart failure. In addition, published and audited prospective data reveals lower or comparable event rates for stroke, vascular complications and major bleeding as compared to IABP and ECMO. The data is detailed in the accompanying chart.
“Abiomed remains focused on our goal of creating the new field of heart recovery and becoming the standard of care for circulatory support. This is an ambitious goal that certainly has challenges with the diffusion of any new break-through innovation, and we are taking specific actions to address these challenges. The long-term outlook for Abiomed remains intact and we are working with physician experts to clarify and educate the community on Impella best practice protocols and clinical outcomes,” said Abiomed Chairman, President and CEO, Michael R. Minogue. “Our product innovation has never been better with the launch of Impella CP® and Impella 5.5™ with SmartAssist®. We have our best ever clinical outcomes with our protocols for high-risk PCI, cardiogenic shock and right heart failure.”
The preliminary unaudited revenue results described in this press release are estimates only and are subject to revision until the company reports its full financial results for the third quarter of fiscal 2020 on February 6, 2020.
These preliminary results are being provided in advance of the company's presentation at the 38th Annual J.P. Morgan Healthcare Conference at the Westin St. Francis Hotel in San Francisco. Michael R. Minogue, Chairman, President and Chief Executive Officer, Abiomed, will present on Monday, January 13, 2020 at 12:00 p.m. PST / 3:00 p.m. EST.
A live webcast of the company's presentation at the conference will be available via the link https://jpmorgan.metameetings.net/events/hc20. The webcast will also be available on the investor section of the company's website at www.abiomed.com. A replay of the webcast will be available for 90 days after the presentation.
FISCAL YEAR 2020 OUTLOOK
The company is updating its fiscal year 2020 revenue guidance to be in the range of $846 million to $877 million, an increase of 10% to 14%, respectively.
EARNINGS CONFERENCE CALL DETAILS
The company will host a conference call to discuss the results at 8:00 a.m. EST on Thursday, February 6, 2020. The conference call releasing full quarterly results will be hosted by Michael R. Minogue, Chairman, President and Chief Executive Officer and Todd A. Trapp, Vice President and Chief Financial Officer.
To listen to the call live, please tune into the webcast via https://edge.media-server.com/mmc/p/v667km57 or dial (855) 212-2361; the international number is (678) 809-1538. A replay of this conference call will be available beginning at 11:00 a.m. EST February 6, 2020 through 11:00 a.m. EST on February 13, 2020. The replay phone number is (855) 859-2056; the international number is (404) 537-3406. The replay access code is 8974195.
ABOUT ABIOMED
Based in Danvers, Massachusetts, USA, Abiomed, Inc. is a leading provider of medical devices that provide circulatory support. Our products are designed to enable the heart to rest by improving blood flow and/or performing the pumping of the heart. For additional information, please visit: www.abiomed.com. Abiomed, Impella, Impella 2.5, Impella 5.0, Impella LD, Impella CP, Impella RP, Impella 5.5, Impella Connect, and SmartAssist are registered trademarks of Abiomed, Inc., and are registered in the U.S. and certain foreign countries. Impella BTR, Impella ECP, CVAD Study, and Automated Impella Controller are pending trademarks of Abiomed, Inc.
FORWARD-LOOKING STATEMENTS
This release contains forward-looking statements, including, without limitation, statements regarding development of Abiomed's existing and new products, the company's progress toward commercial growth, and future opportunities and expected regulatory approvals. All statements, other than statements of historical facts, may be forward-looking statements. These forward-looking statements may be accompanied by such words as “anticipate,” “believe,” “estimate,” “expect,” “forecast,” “intend,” “may,” “plan,” “potential,” “project,” “target,” “should,” “likely,” “will” and other words and terms of similar meaning. The company's actual results may differ materially from those anticipated in these forward-looking statements based upon a number of factors, including, without limitation: the company’s dependence on Impella® products for all of its revenues; the company’s ability to successfully compete against its existing or potential competitors; the acceptance of the company’s products by cardiac surgeons and interventional cardiologists; long sales and training cycles associated with expansion into new hospital cardiac centers; reduced market acceptance of the company’s products due to lengthy clinician training process; the company’s ability to effectively manage its growth; the company’s ability to successfully commercialize its products; the company’s ability to obtain regulatory approvals and market and sell its products in certain jurisdictions; enforcement actions and product liability suits relating to off-label uses of the company’s products; unsuccessful clinical trials or procedures relating to products under development; the company’s ability to maintain compliance with regulatory requirements; the failure of third-party payers to provide reimbursement of the company’s products; the company’s ability to increase manufacturing capacity to support continued demand for its products; the company or its vendors’ failure to achieve and maintain high manufacturing standards; the failure of the company’s suppliers to provide the components the company requires; the company’s ability to expand its direct sales activities into international markets; the outcome of ongoing securities class action litigation relating to our public disclosures and other risks and challenges detailed in the company's filings with the Securities and Exchange Commission (the “SEC”), including the most recently filed Annual Report on Form 10-K and the filings subsequently filed with or furnished to the SEC. Readers are cautioned not to place undue reliance on any forward-looking statements, which speak only as of the date of this release. Unless otherwise required by law, the company undertakes no obligation to publicly release the results of any revisions to these forward-looking statements that may be made to reflect events or circumstances that occur after the date of this release or to reflect the occurrence of unanticipated events.
View source version on businesswire.com: https://www.businesswire.com/news/home/20200113005332/en/
Contacts
Todd Trapp
Vice President and Chief Financial Officer
978-646-1680
ttrapp@abiomed.com
Tom Langford
Director, Communications & Public Relations
978-882-8408
tlangford@abiomed.com
Source: Abiomed, Inc.






