CareStart™ COVID-19 Antigen test kit developed by Access Bio, Inc., using Asahi Kasei’s NanoAct™ cellulose nanobeads, received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA), and its sale in the U.S. has begun.
|
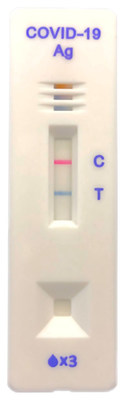
TOKYO, Dec. 8, 2020 /PRNewswire/ -- CareStart™ COVID-19 Antigen test kit developed by Access Bio, Inc., using Asahi Kasei's NanoAct™ cellulose nanobeads, received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA), and its sale in the U.S. has begun. NanoAct™ colored cellulose nanobeads were developed by Asahi Kasei by utilizing its core technology of cellulose processing. They are currently used as labels for lateral flow immunochromatographic assays, mainly for influenza. NanoAct™ was adopted by Access Bio, Inc. for use in CareStart™ COVID-19 Ag as it is available in a wide range of colors and enables better visibility of test lines, which improves usability. The continuing spread of COVID-19 has raised the importance of rapid screening. Demand for rapid test kits is rising because unlike polymerase chain reaction (PCR) tests, they require no special equipment. Access Bio, Inc., the world's leading manufacturer of rapid test kits for malaria, developed CareStart™ COVID-19 Ag to rapidly detect antigens of SARS-CoV-2, the virus which causes COVID-19. Clinical evaluations indicate that CareStart™ COVID-19 Ag provides a sensitivity of 88.4% and specificity of 100%, which is comparable to reverse transcription (RT)-PCR testing. Furthermore, CareStart™ COVID-19 Ag provides results in 10 minutes while PCR generally takes 1 or 2 days. In addition, the availability of NanoAct™ in multi-color variation enables different colors to be shown, such as blue for the test line (T in the figure below) and red for the control line (C), which allows easier judgment as a user-friendly test kit. CareStart™ COVID-19 Ag has also obtained CE Mark certification, and it is scheduled to be launched in Europe, Southeast Asia, and Africa. In addition to various diagnostic test kits for COVID-19 and influenza, NanoAct™ is also expected to be used in multi-testing kits that enable simultaneous testing for several infectious diseases. Such kits are increasingly being developed by many diagnostic kit manufacturers in the U.S., Europe, and Asia. Asahi Kasei will continue to leverage NanoAct™ technology to help prevent the spread of COVID-19 and other infectious diseases. For more information, please contact:
SOURCE Asahi Kasei Corporation |