Delcath Systems, Inc. (Nasdaq: DCTH) today announced the publication of an article entitled “Selective Internal Radiotherapy (SIRT) and Chemosaturation Percutaneous Hepatic Perfusion (CS-PHP) for Metastasized Uveal Melanoma: A Retrospective Comparative Study” in the peer reviewed oncology journal Cancers.
|
|
| [16-October-2023] |
|
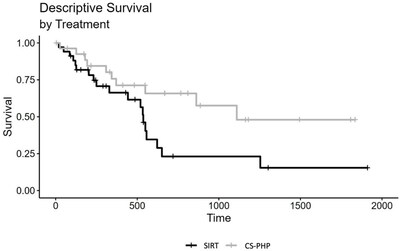
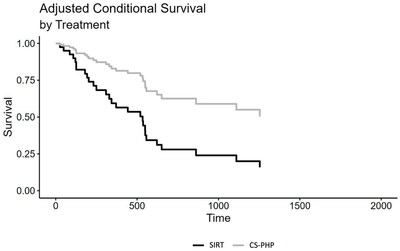
Retrospective Comparison of Two Liver Directed Therapies in Patients with Metastatic Uveal Melanoma Significantly Longer Overall Survival for Patients Treated with Chemosat vs. SIRT (17 and 9.9 months, respectively; p=0.006) NEW YORK, Oct. 16, 2023 /PRNewswire/ -- Delcath Systems, Inc. (Nasdaq: DCTH), an interventional oncology company focused on the treatment of primary and metastatic cancers of the liver, today announced the publication of an article entitled "Selective Internal Radiotherapy (SIRT) and Chemosaturation Percutaneous Hepatic Perfusion (CS-PHP) for Metastasized Uveal Melanoma: A Retrospective Comparative Study" in the peer reviewed oncology journal Cancers. CS-PHP utilizes CHEMOSAT, Delcath's proprietary European CE Marked Hepatic Delivery System (HDS), to administer high-dose chemotherapy (melphalan) to the liver, while controlling systemic exposure and associated side effects during a PHP procedure. Initial findings were previously presented on September 9, 2023 at the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) Annual Meeting held in Copenhagen, Denmark. Uveal melanoma usually shows a liver-dominant metastasis spread and is often treated with liver directed therapies. This retrospective study compared two cohorts of patients with liver dominant uveal melanoma treated at the University Hospitals Tubingen, Germany with multiple cycles of either CS-PHP (N=28) or SIRT (N=34). Patients included in the study were treated between December 2013 and February 2020. Allocation to treatment was determined by a multidisciplinary team. Presence of extrahepatic disease at baseline favored the SIRT group (68% and 41%, CS-PHP and SIRT, respectively), as did the hepatic tumor load (0-25%: 57% and 76%; 26%-50%: 29% and 24%; >50%: 14% and 0%, for CS-PHP and SIRT, respectively). Tumor responses were evaluated by consensus reading by two experienced radiologists. Disease control rates (DCR) were 30% and 18%, for CS-PHP and SIRT, respectively. Median overall survival (mOS) was 516 days for CS-PHP and 300.5 days for SIRT. In a Cox regression model, there was a significant difference between SIRT and CS-PHP treatment, HR = 0.46, CI 95% (0.23; 0.93), p = 0.030. An adjusted Cox regression analysis, including the variables age, sex, presence of extrahepatic metastasis and hepatic load at baseline, also showed a significant effect of choice of treatment, HR = 0.32, CI 95% (0.14; 0.73), p = 0.006. Median progression-free survival (mPFS) was 408.5 days for CS-PHP and 127.5 days for SIRT; the adjusted Cox regression analysis showed a trend favoring CS-PHP (p=0.090). When initially presented at the 2023 CIRSE Annual Meeting held in Copenhagen, Denmark, presenting author Prof. Dr. Med. Gerd Groezinger, stated "Liver directed treatment, including transarterial radioembolization and CS-PHP, is a critical treatment modality for patients with metastatic uveal melanoma, given the longer overall survival seen in the CS-PHP cohort, we conclude that for metastatic uveal melanoma patients, CS-PHP might be the superior liver directed treatment option." "There is a scarcity of comparative studies between liver directed therapies. This peer reviewed publication authored by experienced investigators, supports that the PHP procedure, whether utilizing melphalan delivered by Delcath's CE marked Chemosat or the FDA approved HEPZATO KIT, may be the preferred liver directed treatment option for patients with liver-dominant metastatic uveal melanoma," said Dr. Vojo Vukovic, Delcath's Chief Medical Officer. "We remain committed to making this treatment option available to patients in the US by the end of this year." The full article can be viewed by clicking here. About Chemosat and HEPZATO KIT HEPZATO KIT (melphalan for Injection/Hepatic Delivery System), approved for use in the United States by FDA, is a combination drug/device product which administers HEPZATO (melphalan) directly to the liver through the HDS, which permits higher drug exposure in target tissues while limiting systemic toxicity. HEPZATO KIT is approved in the United States as a liver-directed treatment for adult patients with uveal melanoma with unresectable hepatic metastases affecting less than 50% of the liver and no extrahepatic disease, or extrahepatic disease limited to the bone, lymph nodes, subcutaneous tissues, or lung that is amenable to resection or radiation. HEPZATO KIT Important Safety Information
Most common adverse reactions or laboratory abnormalities occurring with HEPZATO treatment are thrombocytopenia, fatigue, anemia, nausea, musculoskeletal pain, leukopenia, abdominal pain, neutropenia, vomiting, increased alanine aminotransferase, prolonged activated partial thromboplastin time, increased alkaline phosphatase, increased aspartate aminotransferase and dyspnea. Severe peri-procedural complications including hemorrhage, hepatocellular injury, and thromboembolic events may occur via hepatic intra-arterial administration of HEPZATO. HEPZATO is available only through a restricted program under a Risk Evaluation and Mitigation Strategy called the HEPZATO KIT REMS. Myelosuppression with resulting severe infection, bleeding, or symptomatic anemia may occur with HEPZATO. Additional cycles of HEPZATO therapy will be delayed until blood counts have improved. Please see the full Prescribing Information, including BOXED WARNING for the HEPZATO KIT. About Delcath Systems, Inc. Forward Looking Statements Contact: Investor Relations Contact:
SOURCE Delcath Systems, Inc. |
||
Company Codes: NASDAQ-NMS:DCTH |