News
BioSpace data show job postings live increased quarter over quarter, while layoffs fell year over year.
FEATURED STORIES
The FDA’s refusal to review Moderna’s mRNA-based flu vaccine is part of a larger communications crisis unfolding at the agency over the past nine months that has also ensnarled Sarepta, Capricor, uniQure and many more.
The rare disease drugmaker is facing potential competitors for achondroplasia drug Voxzogo. Is a big M&A deal with two approved assets enough to maintain investor interest?
The FDA issued a rare Refusal-to-File letter to Moderna over its mRNA-based influenza vaccine application, in an unusual move that sent the biotech’s shares tumbling.
FROM OUR EDITORS
Read our takes on the biggest stories happening in the industry.
Novo Nordisk and Eli Lilly have been battling head-to-head in an exploding obesity market. They should never have been compared apples to apples.
THE LATEST
After stopping the study early due to strong efficacy, Novo Nordisk released data from the FLOW study showing significant benefits of semaglutide in patients with type 2 diabetes and chronic kidney disease.
The regulator has approved Eyenovia and Formosa’s ophthalmic clobetasol propionate solution to ease eye inflammation and pain after surgery, with a potential $1.3 billion market.
Hugel America has won the FDA’s approval for Letybo, authorized for the treatment of frown lines in adults, which is poised to challenge AbbVie’s blockbuster Botox.
Exosomes show potential to treat myriad conditions, including cancer and inflammation, but experts are divided on whether the therapies are ready for the limelight.
As BridgeBio’s acoramidis inches closer to an FDA approval decision, Bayer on Monday inked a European licensing agreement for the transthyretin amyloid cardiomyopathy treatment.
Pfizer’s oncology strategy to build up its biologics portfolio and dramatically reduce small molecules was influenced by the Inflation Reduction Act’s drug price negotiation provisions.
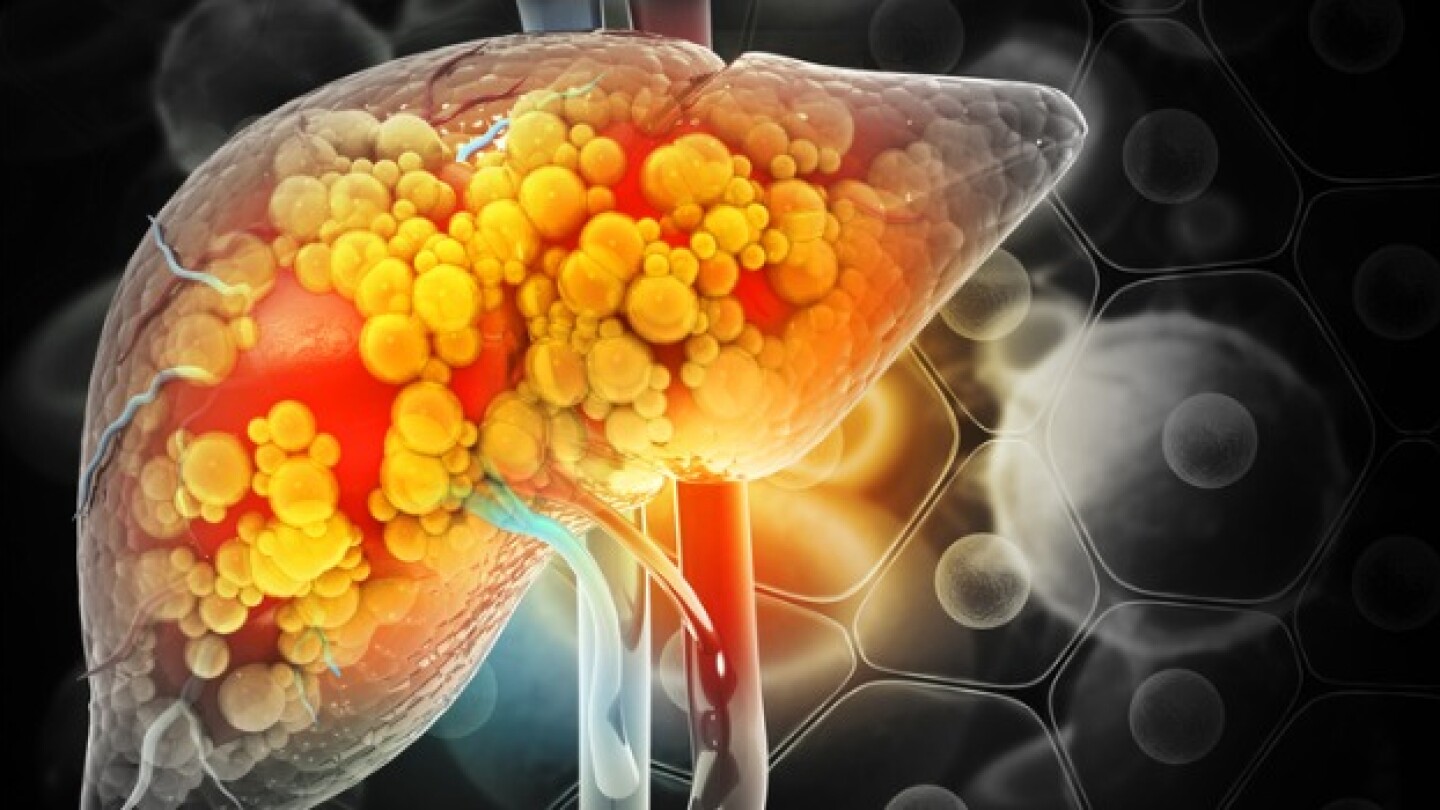
New 96-week data show Akero Therapeutics’ efruxifermin can improve fibrosis by at least one stage without metabolic dysfunction-associated steatohepatitis worsening in more patients versus placebo.
Amid the limitations of current therapies for amyotrophic lateral sclerosis, a new GlobalData report points to novel disease-modifying drug approaches that could transform the space.
Rybrevant has been approved for use with carboplatin and pemetrexed in the first-line treatment of locally advanced or metastatic non-small cell lung cancer with exon 20 insertion mutations in the EGFR gene.
A federal court in Delaware ruled Friday that the pharma company had no “entitlement” to any price above what the buyer is willing to pay.