News
In this episode of Denatured, you’ll be listening to Jane Hughes, President of R&D and Co-founder of Verdiva Bio, and Jon Rees, CEO and Co-founder of MitoRx Therapeutics. We’ll discuss next-generation obesity solutions tackling GLP-1’s muscle loss and adherence challenges, through innovative muscle preservation, oral administration and combination therapy.
FEATURED STORIES
Many scientists-turned-CEOs paradoxically abandon scientific principles when it comes to commercializing their innovations. But applying the scientific method to business decisions can help life science entrepreneurs avoid common pitfalls, attract investment and ultimately bring transformative technologies to market.
FDA vouchers are normally a coveted prize for biopharma companies, but a surprise rejection for Disc Medicine’s rare disease drug has biopharma reconsidering.
PitchBook’s 2025 biopharma VC analysis clocked $33.8 billion in capital dispatched in 2025, mainly to companies with later-stage programs ready to roll into the clinic.
FROM OUR EDITORS
Read our takes on the biggest stories happening in the industry.
The FDA’s refusal to review Moderna’s mRNA-based flu vaccine is part of a larger communications crisis unfolding at the agency over the past nine months that has also ensnarled Sarepta, Capricor, uniQure and many more.
THE LATEST
Bayer will co-create a novel target identification platform that leverages Aignostics’ artificial intelligence technology and proprietary multimodal patient cohorts.
The Chinese biotechs are broadening their collaboration. Hansoh Pharma is licensing Biotheus’ anti-EGFR/cMet bispecific antibody to develop antibody-drug conjugates.
After forging a partnership last year, Astellas is ending the pact with Cartesian Therapeutics and stopping the development of a Pompe disease candidate.
The PD-1 inhibitor Keytruda significantly improved overall survival in a late-stage trial when used with chemoradiotherapy to treat patients with newly diagnosed advanced cervical cancer.
The FDA approved Bristol Myers Squibb’s Breyanzi for chronic lymphocytic leukemia and small lymphocytic leukemia prior to Friday’s adcomm for the company’s other CAR-T therapy, Abecma.
Despite skepticism from FDA reviewers, the Oncologic Drugs Advisory Committee on Thursday strongly supported Geron’s imetelstat for the treatment of anemia in patients with lower-risk myelodysplastic syndromes.
After several delays, BeiGene on Thursday finally secured the FDA’s approval for its PD-1 inhibitor Tevimbra for the treatment of unresectable or metastatic esophageal squamous cell carcinoma.
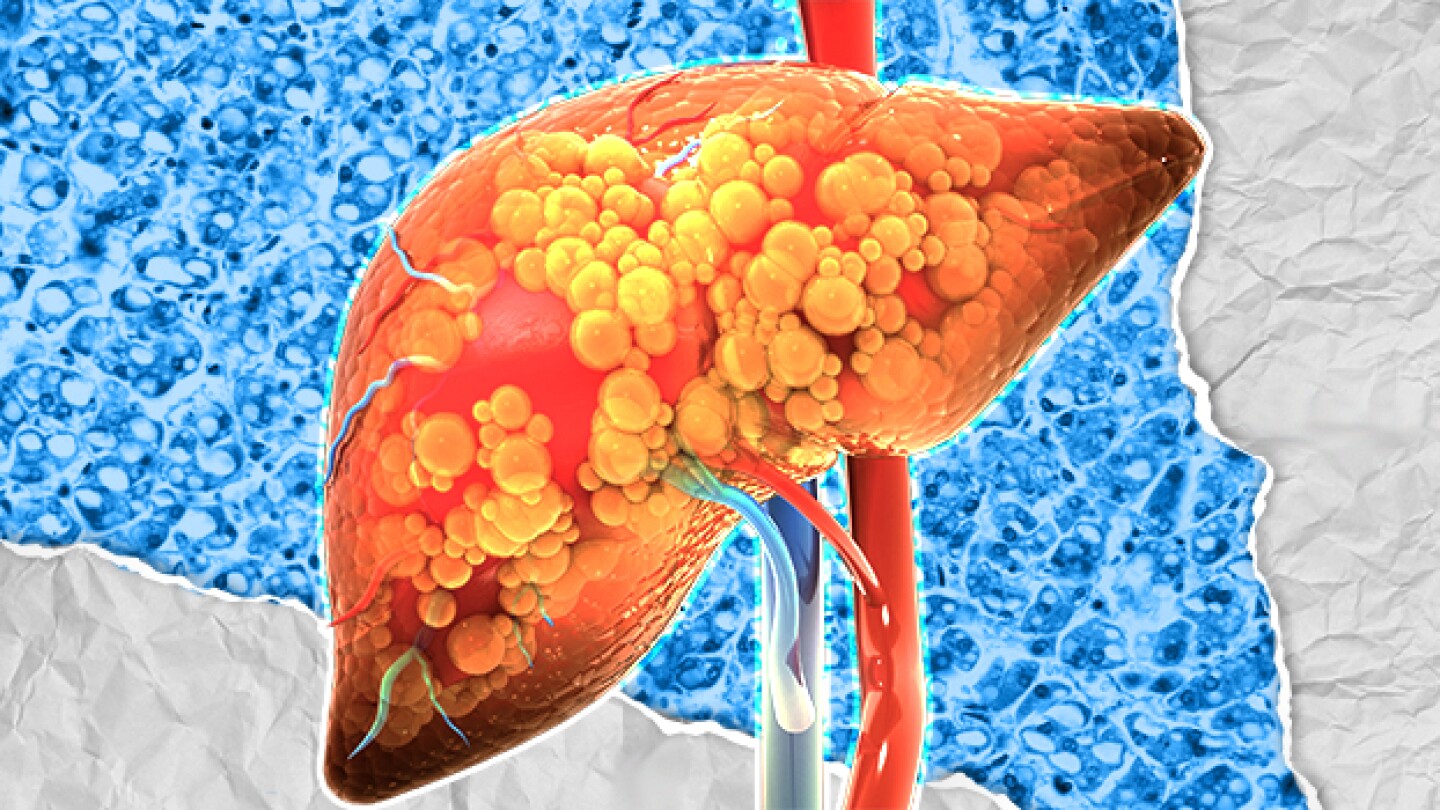
If you’re confused by the NASH versus MASH indication, you’re not alone.
Madrigal Pharmaceuticals’ Rezdiffra (resmetirom) is the first-ever approved therapy for metabolic dysfunction-associated steatohepatitis—a decision experts say could signal a sea change in treatment of the disease.
The U.K. National Institute for Health and Care Excellence on Thursday recommended against funding Vertex Pharmaceuticals’ CRISPR-based sickle cell disease therapy Casgevy unless uncertainties can be cleared up.