News
In this episode of Denatured, you’ll be listening to Jane Hughes, President of R&D and Co-founder of Verdiva Bio, and Jon Rees, CEO and Co-founder of MitoRx Therapeutics. We’ll discuss next-generation obesity solutions tackling GLP-1’s muscle loss and adherence challenges, through innovative muscle preservation, oral administration and combination therapy.
FEATURED STORIES
Many scientists-turned-CEOs paradoxically abandon scientific principles when it comes to commercializing their innovations. But applying the scientific method to business decisions can help life science entrepreneurs avoid common pitfalls, attract investment and ultimately bring transformative technologies to market.
FDA vouchers are normally a coveted prize for biopharma companies, but a surprise rejection for Disc Medicine’s rare disease drug has biopharma reconsidering.
PitchBook’s 2025 biopharma VC analysis clocked $33.8 billion in capital dispatched in 2025, mainly to companies with later-stage programs ready to roll into the clinic.
FROM OUR EDITORS
Read our takes on the biggest stories happening in the industry.
The FDA’s refusal to review Moderna’s mRNA-based flu vaccine is part of a larger communications crisis unfolding at the agency over the past nine months that has also ensnarled Sarepta, Capricor, uniQure and many more.
THE LATEST
An appellate court sided with Regeneron versus Novartis on Monday, agreeing that anti-VEGF pre-filled syringes constitute a distinct market than those sold in vials. The case involves Regeneron’s Eylea and Novartis’ Lucentis eye treatments.
Orchard Therapeutics on Monday secured the FDA’s first approval for an autologous gene therapy to treat the rare metabolic disease metachromatic leukodystrophy in children.
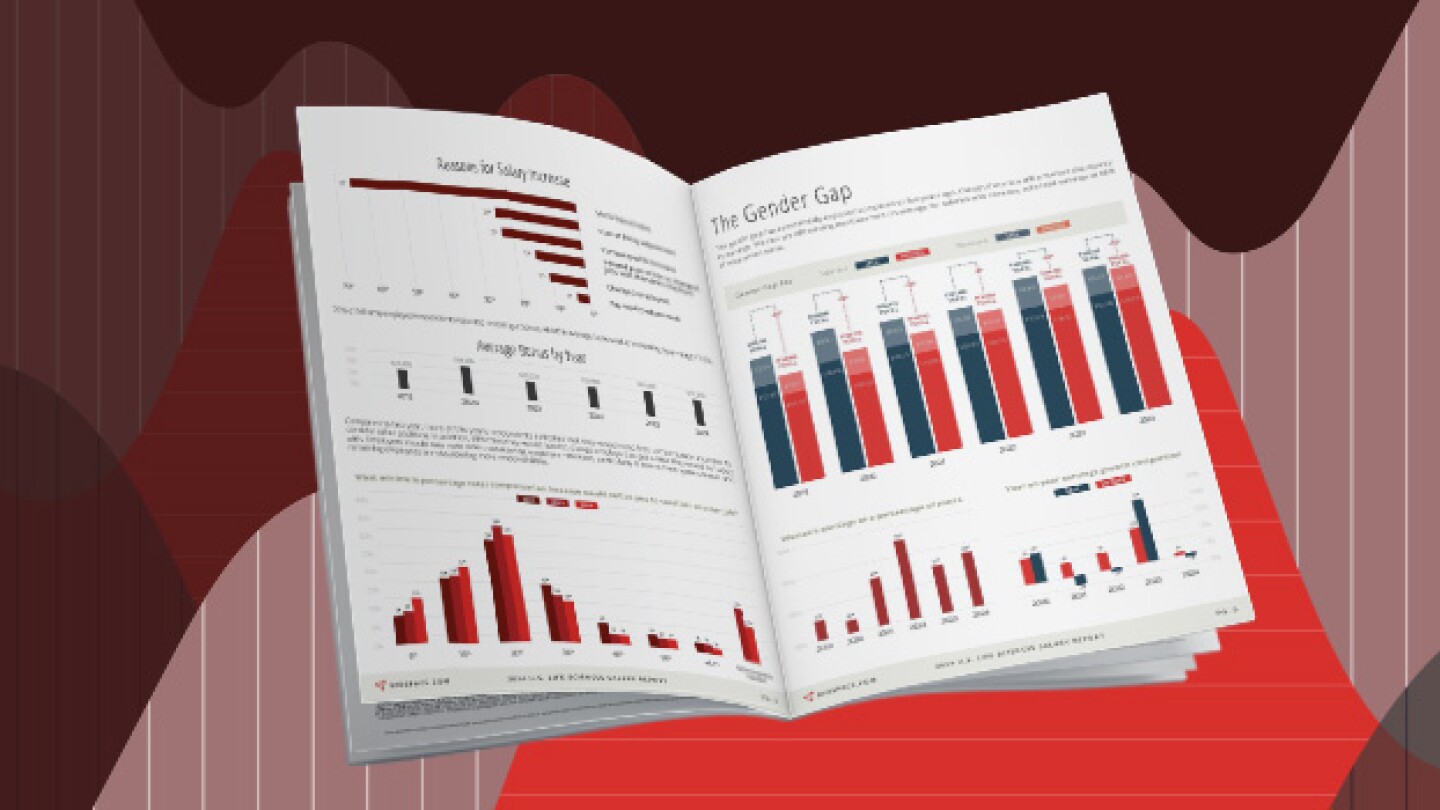
BioSpace’s 2024 Salary Report explores the average salaries and salary trends of life sciences professionals.
AstraZeneca reported Monday that adding Lynparza to Imfinzi improved outcomes in mismatch repair proficient endometrial cancer, more than doubling the median duration of response in patients.
With Boehringer Ingelheim’s announcement earlier this month that it was capping U.S. inhaler costs at $35 per month, AstraZeneca on Monday followed suit.
New late-stage trial results for GSK’s Jemperli show improved overall and progression-free survival in a broader range of endometrial cancer patients, which could lead to a potential label expansion.
Contineum Therapeutics joined the 2024 initial public offering class on Friday with an SEC filing. The biotech will use the IPO proceeds to complete a Phase II trial for its most mature candidate targeting multiple sclerosis.
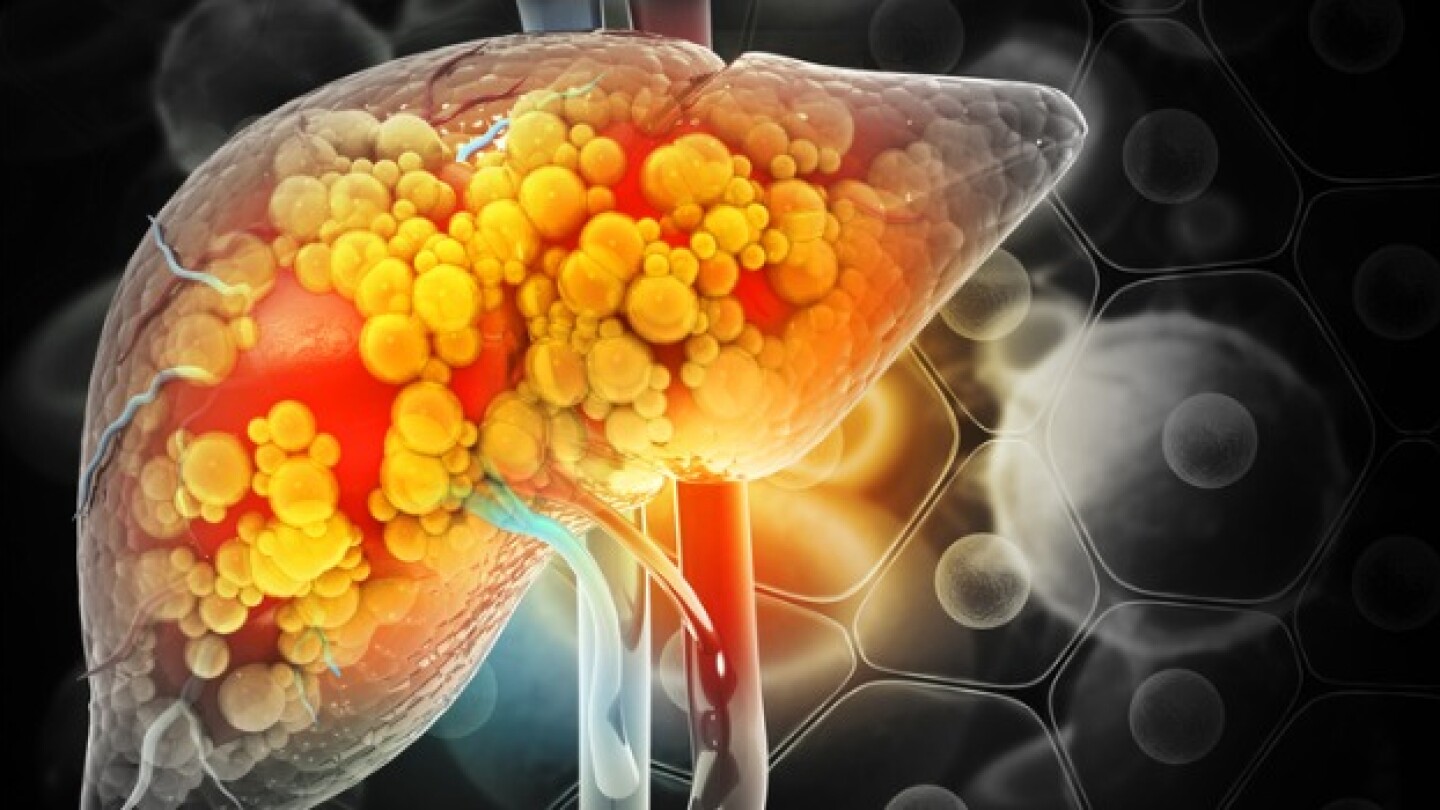
With its FDA approval last week and first-to-market advantage, Madrigal Pharmaceuticals’ Rezdiffra will set the standard for other metabolic dysfunction-associated steatohepatitis candidates in development.
By votes of 11-0 and 8-3, respectively, an FDA advisory committee Friday deemed the risks of early death for both Johnson & Johnson’s Carvykti and Bristol Myers Squibb’s Abecma acceptable.
Asgard Therapeutics, a Swedish gene therapy biotech, has closed a $32 million Series A round with help from prominent pharma players as it prepares for a 2026 IND.