News
FDA vouchers are normally a coveted prize for biopharma companies, but a surprise rejection for Disc Medicine’s rare disease drug has biopharma reconsidering.
FEATURED STORIES
PitchBook’s 2025 biopharma VC analysis clocked $33.8 billion in capital dispatched in 2025, mainly to companies with later-stage programs ready to roll into the clinic.
Long an R&D company that partnered off assets, RNAi biotech Ionis Pharmaceuticals shifted in 2025 to bring two medicines to market alone. Analysts are already impressed—and there’s more to come in 2026.
Regulatory uncertainty is no longer background noise. It is a material investment risk that reshapes how capital is deployed and pipelines are prioritized.
FROM OUR EDITORS
Read our takes on the biggest stories happening in the industry.
The FDA’s refusal to review Moderna’s mRNA-based flu vaccine is part of a larger communications crisis unfolding at the agency over the past nine months that has also ensnarled Sarepta, Capricor, uniQure and many more.
THE LATEST
To help support the launch of Lyfgenia, bluebird bio on Monday entered into a five-year term loan deal with Hercules Capital that will extend the biotech’s cash runway through the first quarter of 2026.
An appellate court sided with Regeneron versus Novartis on Monday, agreeing that anti-VEGF pre-filled syringes constitute a distinct market than those sold in vials. The case involves Regeneron’s Eylea and Novartis’ Lucentis eye treatments.
Orchard Therapeutics on Monday secured the FDA’s first approval for an autologous gene therapy to treat the rare metabolic disease metachromatic leukodystrophy in children.
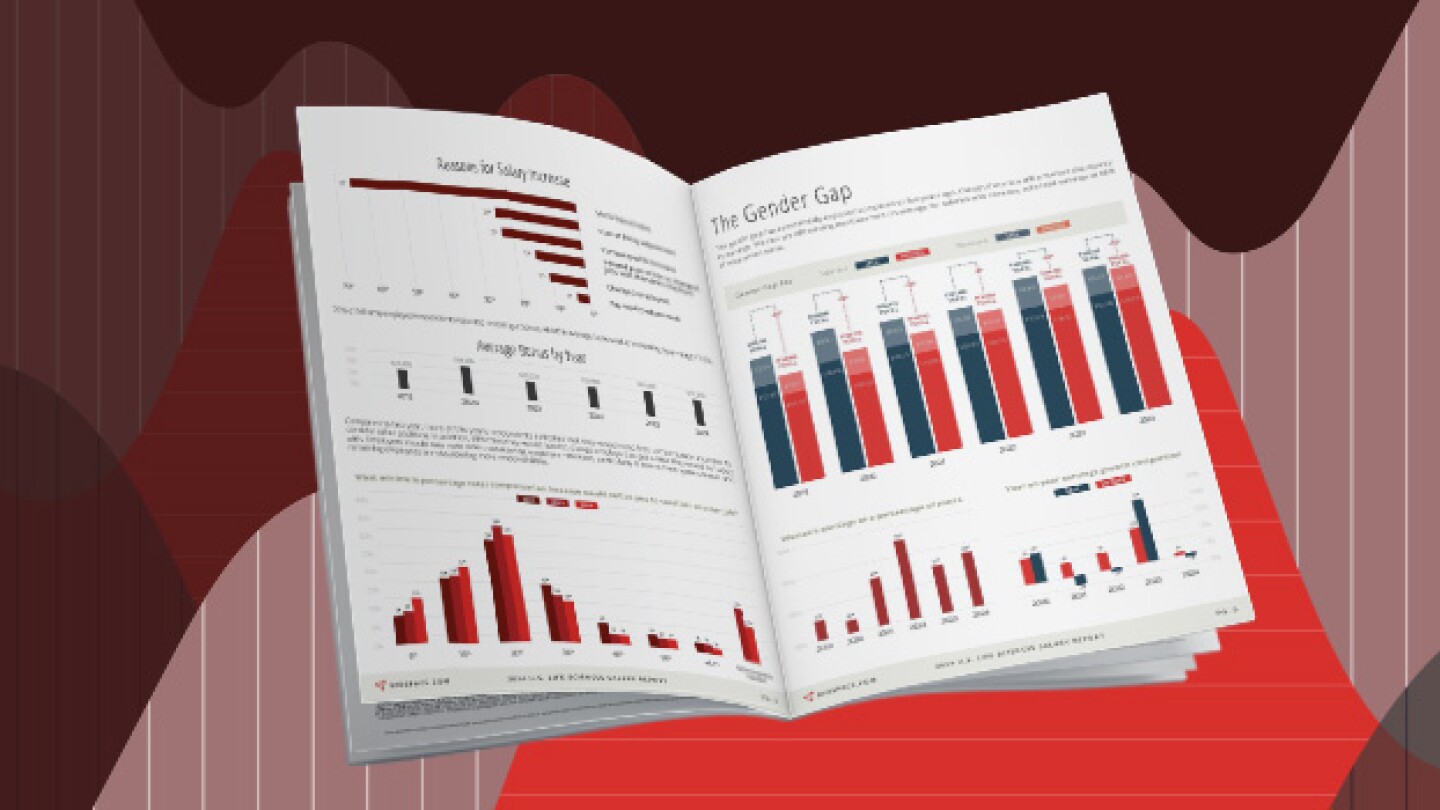
BioSpace’s 2024 Salary Report explores the average salaries and salary trends of life sciences professionals.
AstraZeneca reported Monday that adding Lynparza to Imfinzi improved outcomes in mismatch repair proficient endometrial cancer, more than doubling the median duration of response in patients.
With Boehringer Ingelheim’s announcement earlier this month that it was capping U.S. inhaler costs at $35 per month, AstraZeneca on Monday followed suit.
New late-stage trial results for GSK’s Jemperli show improved overall and progression-free survival in a broader range of endometrial cancer patients, which could lead to a potential label expansion.
Contineum Therapeutics joined the 2024 initial public offering class on Friday with an SEC filing. The biotech will use the IPO proceeds to complete a Phase II trial for its most mature candidate targeting multiple sclerosis.
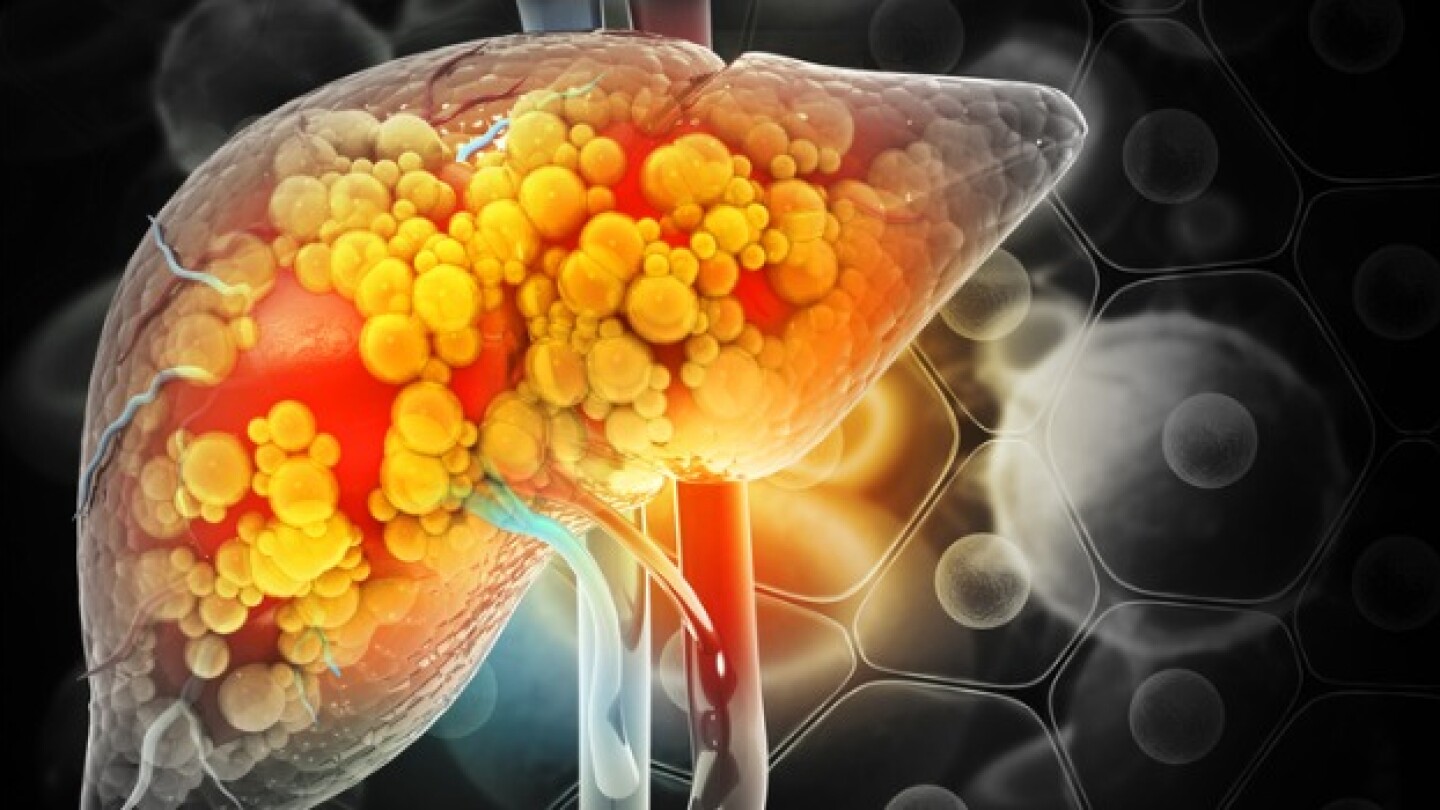
With its FDA approval last week and first-to-market advantage, Madrigal Pharmaceuticals’ Rezdiffra will set the standard for other metabolic dysfunction-associated steatohepatitis candidates in development.
By votes of 11-0 and 8-3, respectively, an FDA advisory committee Friday deemed the risks of early death for both Johnson & Johnson’s Carvykti and Bristol Myers Squibb’s Abecma acceptable.