News
The drug, a small molecule protein inhibitor, brought in $132 million in the first quarter, missing consensus estimates by 17%.
FEATURED STORIES
Experts say approval of Lykos Therapeutics’ MDMA capsules for post-traumatic stress disorder would open the door to further research into psychedelic-assisted therapies.
AstraZeneca last week set another ambitious goal, this time with plans to nearly double its total revenue by the end of the decade. However, it’s easier said than done, according to analysts.
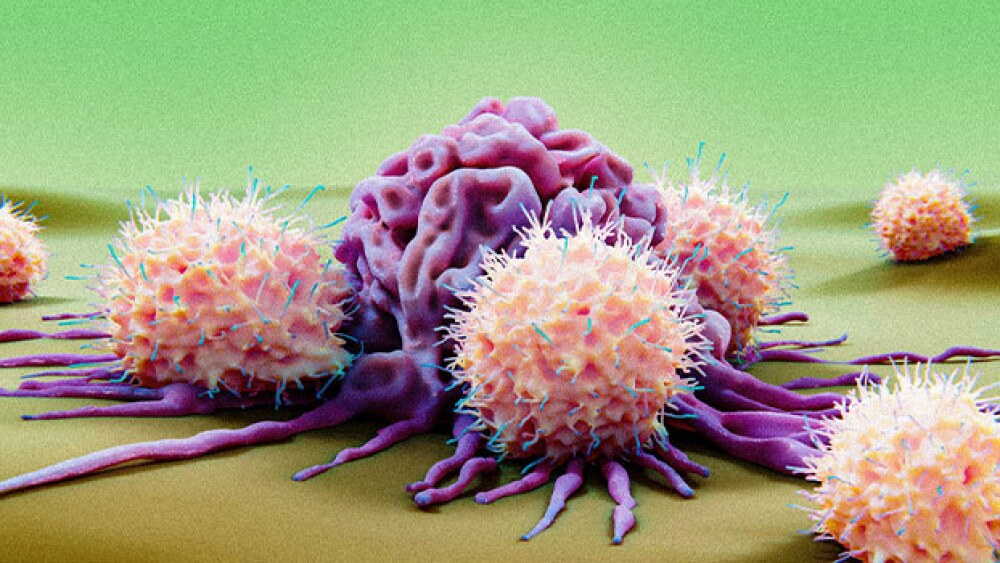
While NK cell therapies can potentially avoid the serious side effects sometimes seen with CAR T cell therapies, experts say durability may stall their path to the market.
Job Trends
The Janssen Pharmaceutical Companies of Johnson & Johnson announced that the U.S. FDA has granted accelerated approval of TALVEY™, a first-in-class bispecific antibody for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 antibody.
FROM OUR EDITORS
Read our takes on the biggest stories happening in the industry.
Unpredictable communication and a lack of transparency are eroding the industry’s and the public’s trust. The FDA, experts agree, needs to take control of the narrative.
THE LATEST
After the Phase II failure of its lead asset from Cerevel, AbbVie is resetting expectations and narrowing the clinical program to an adjunct approach—for now.
ImmunityBio will part with 10 employees this quarter. Last fall, it cut 31 employees. The moves come as the biotech works to advance Anktiva in non-small cell lung cancer.
While the former Biden administration showcased the Inflation Reduction Act as a key victory in the fight over high drug prices in the U.S., Trump has so far been mum on how the controversial law could evolve in the coming years.
Fractyl Health is deprioritizing a type 2 diabetes trial in favor of a pivotal study of its endoscopy treatment Revita and parting with around 22 employees in an attempt to extend its runway into 2026.
Biogen’s effort to buy Sage against the board’s wishes and a long-time effort by investor Alcorn to scuttle Aurion’s IPO underscore the cutthroat nature of biopharma dealmaking.
The approval of Axsome Therapeutics’ Symbravo for migraine with or without aura came alongside the greenlight for Vertex’s non-opioid treatment Journavx.
Novartis was among the most prolific pharma dealmakers in 2024, a trend that it expects to continue with more bolt-on deals this year to set up for sustainable long-term growth.
Senators on the Health, Education, Labor and Pensions committee were critical of Kennedy’s long history as an anti-vaccine campaigner.
Blackstone joins other big investors such as ARCH Venture Partners and Bain Capital Life Sciences in pumping billions of dollars into the industry.
The greenlight for Journavx (suzetrigine), which comes on the heels of a $7.4 billion opioid settlement, could spark momentum in the fledgling non-opioid pain space.