Difamilast is a novel atopic dermatitis compound discovered by Otsuka.
Fairfield, New Jersey-based Medimetriks Pharmaceuticals announced that its partner Otsuka Pharmaceuticals UK reported positive data in two Phase III clinical trials of MM36 (difamilast) in adult and pediatric patients with mild to moderate atopic dermatitis. The studies were conducted in Japan.
Difamilast is a novel atopic dermatitis compound discovered by Otsuka. It inhibits phosphodiesterase 4 (PDE4). These types of drugs appear to improve atopic dermatitis by suppressing the production of chemical mediators like proinflammatory cytokines and other anti-inflammatory mechanisms.
The two companies entered into the licensing deal in February 2016. Medimetriks holds the exclusive development and commercialization rights for the drug in the U.S. and Puerto Rico, as well as manufacturing rights. Under the terms of the deal, Medimetriks paid Otsuka $22 million up front and additional milestones through NDA approval. Medimetriks had funded and handled Phase III trials in the U.S.
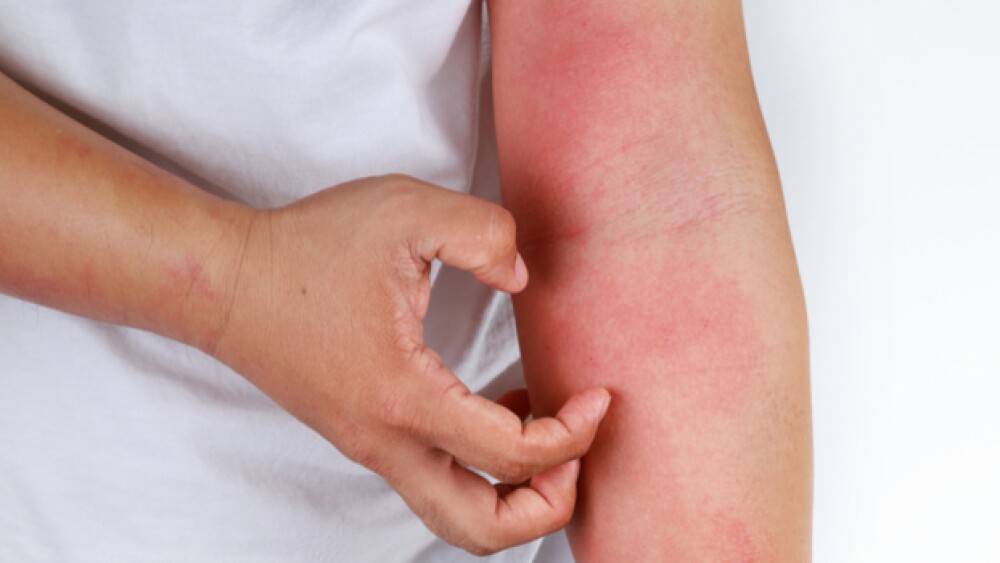
Atopic dermatitis is a chronic inflammatory skin condition resulting in red, swollen and cracked skin that itches. Onset occurs most often between three and six months of age, with about 60% of patients developing it in the first year of life and 90% by the age of five years. Most resolve it during childhood, although 10% to 30% have it their entire lives. A small percentage develops first symptoms as adults. About 18 million people in the U.S. have atopic dermatitis. Current treatments include topical corticosteroids and topical calcineurin inhibitors.
“We are excited about the results announced today by Otsuka and believe these results further validate the potential for MM36 (difamilast) as a best-in-class treatment for patients suffering from mild to moderate atopic dermatitis,” said Bradley Glassman, chairman and chief executive officer of Medimetriks. “We are preparing for our U.S. Phase III MM36 (difamilast) trials and are committed to our goal of helping improve the lives of atopic dermatitis patients in the United States.”
The two clinical trials were conducted as multicenter, randomized, double-blind, vehicle-controlled, parallel-group studies in Japan. In the study in adults, patients received 1% difamilast ointment or the placebo (vehicle) twice a day for four weeks. In the pediatric study, 0.3% or 1% difamilast ointment or the vehicle was also applied twice a day for four weeks. Therapeutic effects were evaluated using the Investigator’s Global Assessment (IGA), a standard method for clinical trials in atopic dermatitis that used severity scoring to determine systemic symptoms.
In both trials, the IGA success rates, the primary endpoints were the proportion of patients whose IGA score was 0 (clear) or 1 (almost clear) and with improvement by at least two grades, were higher in the active-drug-treatment groups than in the placebo groups, and the differences were statistically significant. There were no observed major adverse events.
Otsuka indicated it plans to analyze the data more and eventually release it at a scientific conference.
Otsuka also announced at the same time that it and the National Cancer Center Japan in Tokyo had jointly developed a comprehensive genomic profiling test for hematologic cancers. It includes almost all categories of hematologic malignancies, including acute myeloid leukemia, myelodysplastic syndrome, and myeloproliferative neoplasm, acute lymphoblastic leukemia, lymphoma, and myeloma, and congenital bone marrow failure syndromes. The assay will be used to provide patients and physicians detailed information on diagnosis, treatment and prognostic prediction.