Drug Delivery
The Denton site is part of a network of manufacturing plants Novartis is building across the U.S. to make cancer drugs that must be shipped to patients quickly.
FEATURED STORIES
The limited supply of this common reagent is set to drive drug prices higher, but there are ways for companies to lessen the impact.
Suppliers are investing in production to support deals with AstraZeneca, Bayer and other drugmakers that are advancing radioisotope-based cancer therapies.
After suffering in the wake of expired tax incentives for pharmas, the island is trying to take advantage of geopolitics to grow its drug manufacturing sector.
Subscribe to Manufacturing Brief!
Weekly insight into the biggest trends in biopharma manufacturing
THE LATEST
Nucleus RadioPharma’s two sites are meant to help address the industry’s lack of manufacturing and development capabilities, as well as geographic limitations associated with the short half-life of many radioactive components.
With Friday’s ruling by New Jersey District Judge Zahid Quraishi, Novartis joins a growing list of pharmaceutical companies that have failed in their legal challenges to the Inflation Reduction Act.
United States Pharmacopeia is recruiting expert volunteers from academia, industry, regulatory and healthcare to develop, revise and approve medicine, dietary supplement and food ingredient standards and solutions used in more than 150 countries to improve global public health. The volunteers will serve from 2025 to 2030.
J&J beat expectations this week to launch the Q3 earnings season; a study about children treated with bluebird bio’s Skysona comes at a bad time for the company; Sen. Warren calls for scrutiny of Novo’s purchase of Catalent; and other news.
European CDMO Ardena will buy Catalent’s oral solids manufacturing facility in Somerset, N.J.
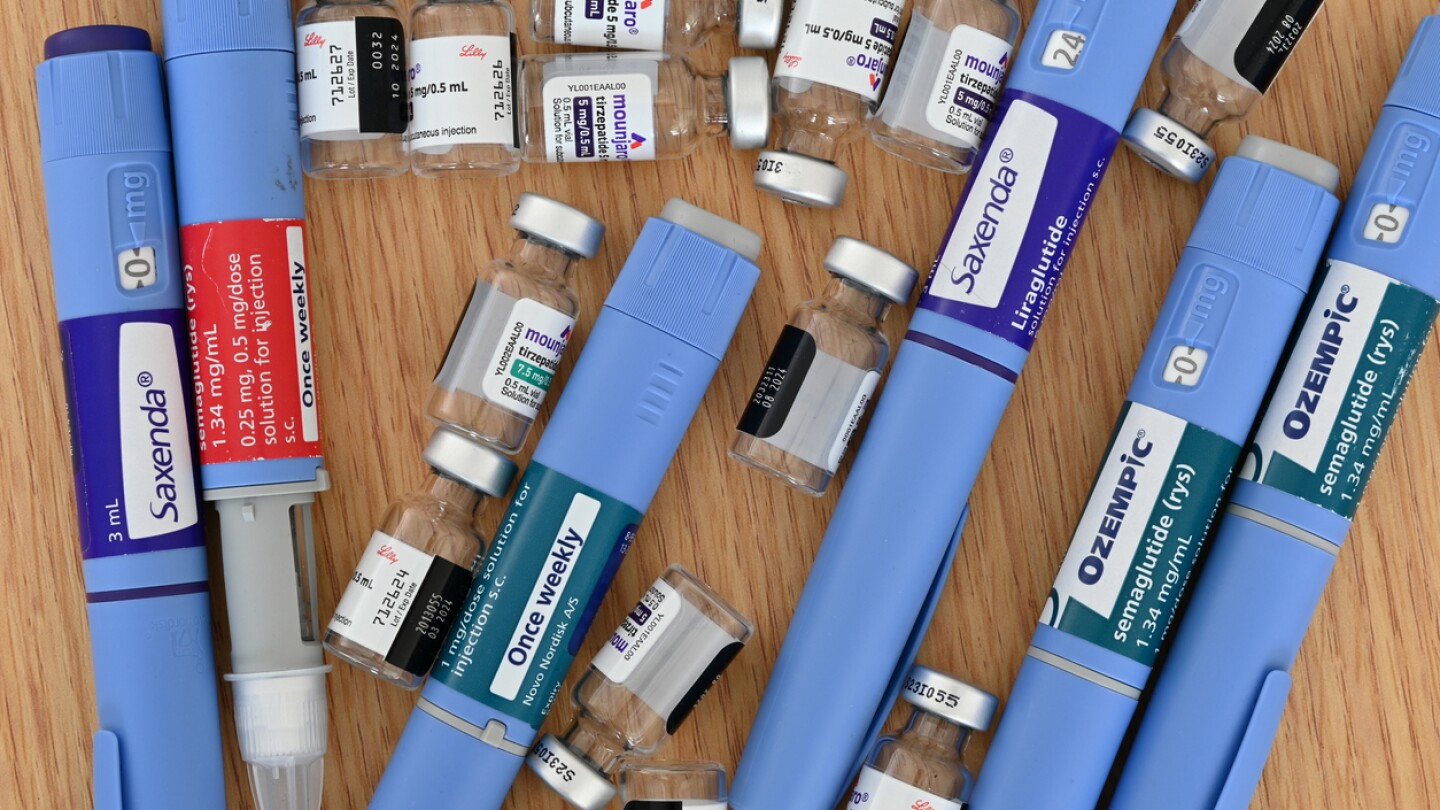
While the regulator conducts another review into the supply of Eli Lilly’s tirzepatide, compounders will be able to continue selling their own remixed versions of the blockbuster drug.
While ex vivo genome editing results in highly effective cell therapies, it can lead to off-target effects. Caribou Biosciences has come up with a novel approach for potentially more precise gene editing compared to all-RNA guides.
Large pharmaceutical companies were out in force at this week’s 2024 Cell & Gene Meeting on the Mesa, as they look to expand their presence in the industry.
WuXi AppTec looks to unload its Philadelphia manufacturing sites and WuXi Biologics slows its rapid expansion in the U.S. as the companies await the Senate’s review of the BIOSECURE Act that threatens to cut them off from U.S. biopharma.
Scaling GLP-1 manufacturing capacity remains a key priority for the pharma industry, to help supply catch up with the insatiable demand for weight loss drugs.