All News
Despite strong demand for weight-loss drugs, the lack of Medicare coverage is potentially interfering with prescription and dispensing rates of anti-obesity medications for elderly adults, according to a new study.
Bristol Myers Squibb’s $4.8 billion acquisition of Mirati pays off with strong data from the KRYSTAL-12 study of Krazati, showing that the KRAS inhibitor significantly improves progression-free survival.
Avalo Therapeutics rolled the dice on a big pivot Wednesday, acquiring AlmataBio and focusing on the biotech’s ex-Eli Lilly hidradenitis suppurativa candidate over its existing assets.
Gamida Cell, whose cell therapy for blood cancer was approved last year by the FDA, is being taken private and restructuring due to liquidity constraints.
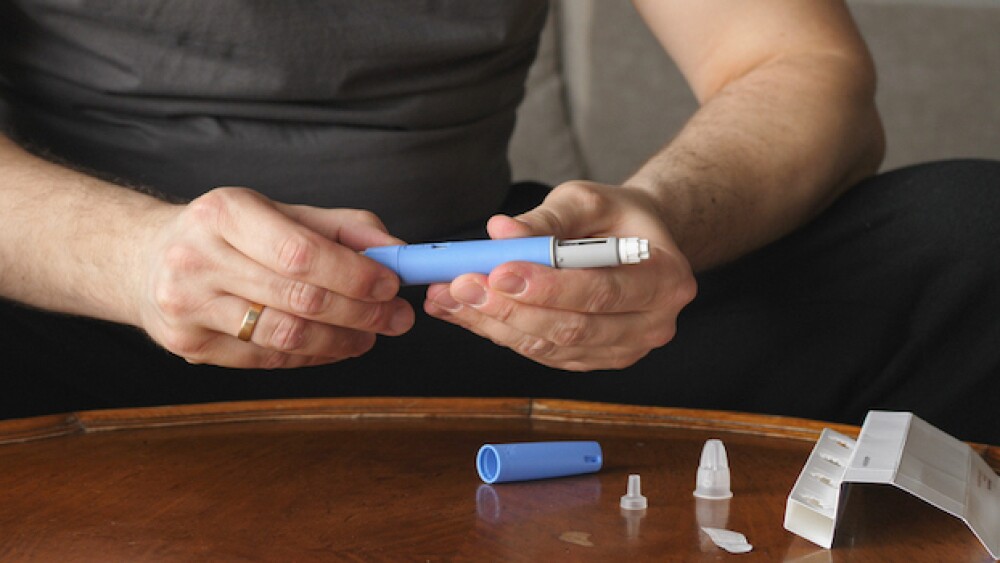
Sanders says he wants Novo Nordisk to “do the right thing” and lower the costs of Ozempic and Wegovy. But only the Inflation Reduction Act can achieve that.
U.S. intelligence officials have informed senators that China-based biotech WuXi AppTec transferred an American client’s intellectual property to the Chinese government without consent, reports Reuters.
Citing the need for more time to review a major amendment, the FDA is pushing the target action date for the investigational aldose reductase inhibitor in the treatment of classic galactosemia to Nov. 28, 2024.
Gilead Sciences’ Vemlidy on Thursday secured a label expansion from the FDA, allowing the antiviral drug’s use to treat chronic hepatitis B in pediatric patients aged six years and older.
Data from a late-stage study showed that Bristol Myers Squibb’s Zeposia (ozanimod) was unable to significantly improve clinical remission in adult patients with moderate to severe active Crohn’s disease.
The Swiss contract manufacturer’s cash deal for Roche’s facility in Vacaville, California, is one of the world’s largest manufacturing sites for biologics—a major growth driver for Lonza and other CDMOs.
Now in Phase III with its small molecule orforglipron, Eli Lilly is leading the oral GLP-1 race against Novo Nordisk, Pfizer, Roche and others.
Altimmune on Wednesday said it is ending development of HepTcell, a hepatitis B candidate, following disappointing trial results as it focuses on obesity and metabolic dysfunction-associated steatohepatitis.
The pharmaceutical industry is facing critical attention, particularly around drug pricing and development costs. Drug development cost is about 10% of the total healthcare spend in the United States. Broader issues such as local monopolies, utilization, unit, and costs and local monopolies, politics and a fragmented payer system contribute to the increasingly high costs to patients.
As the Biden administration calls for more-expansive drug pricing controls, it’s important to reflect on how we’re arriving at expected outcomes.
Stoke Therapeutics plans to offer up more than 5.5 million shares of its common stock following positive Phase I/IIa data for its Dravet syndrome candidate.
Keytruda can now be used in the European Union for patients with resectable non-small cell lung cancer at high risk of recurrence in combination with platinum chemotherapy, then continued as a monotherapy afterwards.
April kicks off with three FDA target action dates, including one that could potentially set the stage to move CAR-T therapies into earlier lines of treatment.
After an initial rejection due to safety issues, followed by a dispute and deferred actions, Akebia Therapeutics on Wednesday finally won the FDA’s nod for vadadustat as a treatment for anemia caused by chronic kidney disease.
Continuing 2024’s biotech initial public offering rally, Boundless Bio will debut Thursday on the Nasdaq with the proceeds used to advance its pipeline of extrachromosomal DNA cancer assets.
Citing a JAMA study that found Ozempic could be profitably produced at under $5 per month, Senator Bernie Sanders on Wednesday called on Novo Nordisk to lower prices for the diabetes treatment and the weight-loss drug Wegovy.