All News
Johnson & Johnson announced Thursday it is paying $850 million in cash, plus a potential milestone payment, for privately held biotech Proteologix and its atopic dermatitis-focused bispecific antibody candidates.
Roche’s $2.7 billion acquisition of Carmot Therapeutics in December 2023 appears to be paying off as its investigational GLP-1/GIP receptor agonist induced strong weight loss in a Phase Ib study.
Belgian biotech Galapagos is teaming with Blood Centers of America to help deploy its decentralized CAR-T therapy manufacturing platform closer to treatment centers across the U.S.
With a June 4 FDA advisory committee meeting looming, the Institute for Clinical and Economic Review has raised concerns about Lykos Therapeutics’ trials of its MDMA-assisted therapy in post-traumatic stress disorder.
Following back-to-back approvals in lymphocytic leukemia, Bristol Myers Squibb’s CAR-T therapy Breyanzi on Wednesday won the FDA’s green light for relapsed or refractory follicular lymphoma.
While biopharma professionals cited age discrimination as an issue in a new BioSpace report, it’s not the only factor affecting older and younger people’s job searches.
Looking for software development jobs in the biopharma industry? Check out these five top companies hiring life sciences professionals like you.
With medicine becoming more specialized and clinical trial protocols increasingly complex, we need to think about how to drive efficiencies to overcome these challenges and keep up the pace of research.
Armed with a $300 million commitment from Blackstone Life Sciences and a former Merck monoclonal antibody, Uniquity Bio is starting Phase II clinical trials in asthma and chronic obstructive pulmonary disease.
The weight-loss drug bonanza continued in the first quarter of 2024 for Novo Nordisk and Eli Lilly, as Amgen also posted strong results, while Biogen and BMS struggled early in the financial year.
While the FTC continues to review its $16.5 billion buy of Catalent, Novo Holdings announced Wednesday it has acquired a majority stake in Single Use Support, an Austrian life sciences tools company.
With more than half a billion dollars in the new life sciences fund, Sands Capital is looking to finance private companies working to transform the diagnosis and treatment of diseases with large unmet medical needs.
Following an initial rejection in 2023, Ascendis Pharma on Tuesday said it faces another regulatory bump in the road for TransCon PTH with a three-month delay in the ongoing FDA review.
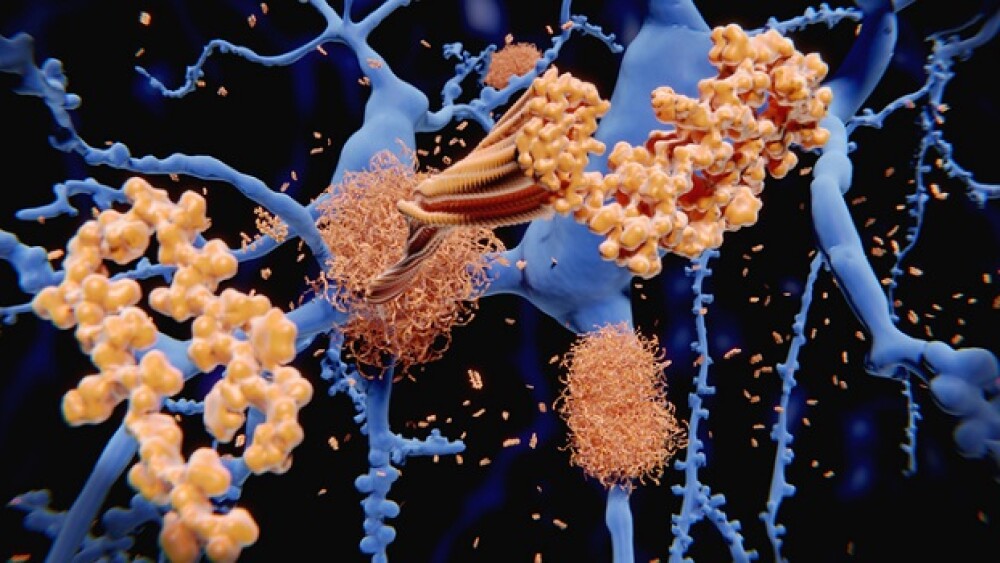
After missing their initial target in March 2024, Eisai and Biogen have initiated a rolling BLA for a subcutaneous maintenance formulation of Leqembi, which could offer a more convenient dosing schedule for Alzheimer’s disease patients.
Bayer joined BMS in announcing major overhaul; Takeda drops up to $2 billion for an anti-amyloid drug from AC Immune; and BioSpace reflects on last week’s ASGCT meeting—the good, the bad and the ugly.
Learn how to discuss career gaps and how to be a great hiring manager and interviewer.
Dynavax Technologies announced Tuesday that the FDA issued a Complete Response Letter to the company’s sBLA to include a four-dose regimen of Heplisav-B vaccine for adult hemodialysis patients.
Applications of the technology range from data collection to drug design to raising the alarm on product safety, but its adoption is also creating some anxiety.
In Tuesday’s first-quarter 2024 financial results, Bayer reported a slight drop in sales amid its sweeping companywide restructuring that resulted in workforce reductions, while lowering its full-year earnings outlook.
With Monday’s agreement, AbbVie joins the industry’s growing interest in next-generation psychiatric therapies and looks to leverage Gilgamesh Pharmaceuticals’ research platform to discover novel neuroplastogens.