CASTLE ROCK, Colo., Oct. 27, 2014 /PRNewswire/ -- Venaxis, Inc. (Nasdaq: APPY), an in vitro diagnostic company focused on obtaining FDA clearance for and commercializing its CE Marked APPY1 Test, a rapid blood test for aiding in identifying children, adolescent, and young adult patients in the emergency room who are at low probability for appendicitis, today announced the company's pivotal APPY1 Test clinical trial data were presented at the American College of Emergency Physicians Scientific Assembly 2014 (ACEP14). The clinical trial involved 29 U.S. hospital sites recruiting patients aged 2 20 from January 2013 through January 2014. Results were presented by Lead Principal Investigator Dr. David S. Huckins, M.D.
1887 patients aged 2 to 20 years with suspected acute appendicitis were included in the study. The results of the study showed the APPY1 Test exhibited a sensitivity of 96.9% (95% CI, 94.9-98.1%), a negative predictive value of 97.3% (95% CI, 95.5-98.3%), a negative likelihood ratio of 0.08 (95% CI, 0.05-0.14), and a specificity of 37.8% (95% CI, 35.5-40.4%) for acute appendicitis. Prevalence of disease was 25.3%. The APPY1 Test correctly identified 533 of 1409 (37.8%) patients who did not have appendicitis with 15 (3.1%) false negatives among 478 patients with acute appendicitis. Among patients without appendicitis, 32% (136/431) who underwent a computed tomography (CT) were correctly identified by negative APPY1 Test results. The APPY1 Test is under FDA review and is currently not available for sale in the U.S. The following represents the clinical flowchart pathway with respect to patient disposition:
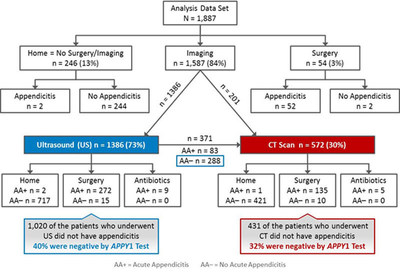
"We want to express our sincere appreciation to all of our investigators who worked extremely hard to complete this important study," said Steve Lundy, President and CEO of Venaxis. "The increased risk of radiation induced cancers associated with CT scans is of particular concern in younger patients, due to their size, radio-sensitivity, and longer spans of time to develop these cancers. We believe the results of our trial demonstrate our test to be of important future value as a means to potentially help reduce radiation exposure in children and improve patient flow in crowded emergency departments."
About Venaxis, Inc.
Venaxis, Inc. is an in vitro diagnostic company focused on the clinical development and commercialization of its CE Marked APPY1 Test, the Company's rapid blood based test for appendicitis. This unique appendicitis test has projected high sensitivity and negative predictive value and is being developed to aid in the identification of patients at low probability for acute appendicitis, allowing for more conservative patient management. The APPY1 Test is being developed initially for pediatric, adolescent, and young adult patients with abdominal pain, as this population is at the highest risk for appendicitis and has the highest risk of long-term health effects associated with CT imaging. While FDA clearance is being sought, a limited commercial launch for the APPY1 Test is ongoing in select European countries. For more information, visit www.venaxis.com.
Forward-Looking Statements
This press release includes "forward-looking statements" of Venaxis, Inc. ("Venaxis") as defined by the Securities and Exchange Commission ("SEC"). All statements, other than statements of historical fact, included in this press release that address activities, events or developments that Venaxis believes or anticipates will or may occur in the future are forward-looking statements. These statements are based on certain assumptions made based on experience, expected future developments and other factors Venaxis believes are appropriate in the circumstances. Such statements are subject to a number of assumptions, risks and uncertainties, many of which are beyond the control of Venaxis. Investors are cautioned that any such statements are not guarantees of future performance. Actual results or developments may differ materially from those projected in the forward-looking statements as a result of many factors, including our ability to obtain FDA clearance or approval, maintain CE Marking, cost effectively manufacture and generate revenues from the APPY1 Test at a profitable price point, execute agreements required to successfully advance the company's objectives, retain the management team to advance the products, overcome adverse changes in market conditions and the regulatory environment, obtain and enforce intellectual property rights, and realize value of intangible assets. Furthermore, Venaxis does not intend (and is not obligated) to update publicly any forward-looking statements. The contents of this press release should be considered in conjunction with the risk factors contained in Venaxis' recent filings with the SEC, including its Form 10-K for the year ended December 31, 2013, filed with the SEC on March 28, 2014.
Venaxis is a registered trademark and APPY1 is a trademark of Venaxis, Inc.
Contact Jed Mahan, jmahan@venaxis.com; (303) 794-2000 Ext. 255
Photo - http://photos.prnewswire.com/prnh/20141027/154636
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/venaxis-appy1-trial-results-presented-by-dr-david-s-huckins-md-at-acep14-308815700.html
SOURCE Venaxis, Inc.




