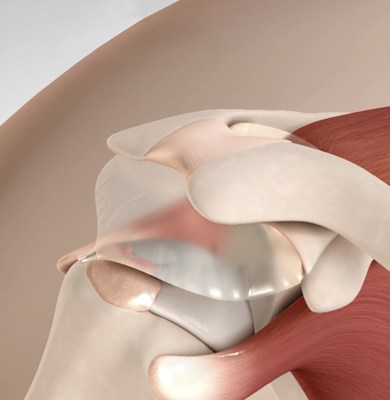
Stryker announced the FDA clearance of the first balloon implant for arthroscopic treatment of massive irreparable rotator cuff tears.
The InSpace balloon implant is a breakthrough solution for arthroscopic treatment of massive irreparable rotator cuff tears that is efficient and provides sustained patient improvements (NCT02493660)
KALAMAZOO, Mich., July 13, 2021 /PRNewswire/ -- Stryker announced today the FDA clearance of the first balloon implant for arthroscopic treatment of massive irreparable rotator cuff tears (MIRCTs)*. InSpace provides a new option for surgeons in their shoulder continuum of care that allows them to better meet the needs of their patients. This ground-breaking technology was acquired from OrthoSpace, Ltd. in 2019 and is the first of its kind in the U.S. market. The InSpace balloon implant has a long successful clinical history of over 10 years and 29,000 balloons implanted outside of the US, as well as the Level I study conducted across North America.
"Current strategies treating massive irreparable rotator cuff tears often present a challenge to surgeons and may require long and frustrating rehabilitation processes for patients," said the lead investigator in the clinical study, Dr. Nikhil Verma, M.D. "The results of the study demonstrate the InSpace balloon is a 'game-changer' and presents a shorter, less invasive option that may enable sustained, clinically meaningful improvements in shoulder function and symptoms."
The InSpace balloon implant is designed to restore the subacromial space without requiring sutures or fixation devices and has been demonstrated to improve shoulder motion and function.
"We are extremely excited about the clearance of InSpace because it provides a new surgical option for surgeons to address their unmet MIRCT needs in the shoulder continuum of care," said Matt Moreau, Stryker's Sports Medicine Vice President and General Manager. "We are committed to the advancement of shoulder arthroscopy, and InSpace offers a unique opportunity for us to better partner with our customers on their clinical objectives to improve patient outcomes around a very challenging pathology in the shoulder."
Learn more about the InSpace balloon implant at www.stryker.com/inspace
About Stryker
Stryker is one of the world's leading medical technology companies and, together with its customers, is driven to make healthcare better. The company offers innovative products and services in Orthopaedics, Medical and Surgical, and Neurotechnology and Spine that help improve patient and hospital outcomes. More information is available at www.stryker.com.
Media contact
Kara Rasmussen
Director, Communications and PR
kara.rasmussen@stryker.com
Phone: 408 529 7512
*The InSpace™ subacromial tissue spacer system is indicated for the treatment of patients with massive, irreparable full-thickness torn rotator cuff tendons due to trauma or degradation with mild to moderate gleno-humeral osteoarthritis in patients greater than or equal to 65 years of age whose clinical conditions would benefit from treatment with a shorter surgical time compared to partial rotator cuff repair.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/stryker-announces-the-fda-clearance-of-the-first-biodegradable-subacromial-balloon-spacer-filling-a-gap-in-the-shoulder-continuum-of-care-301332052.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/stryker-announces-the-fda-clearance-of-the-first-biodegradable-subacromial-balloon-spacer-filling-a-gap-in-the-shoulder-continuum-of-care-301332052.html
SOURCE Stryker