A first-in-class clinical trial will initiate this quarter to determine if a checkpoint agonist can down-regulate activated T cells to help reduce inflammation and tissue damage in patients with moderate-to-severe ulcerative colitis (UC).
A first-in-class clinical trial will initiate this quarter to determine if a checkpoint agonist can down-regulate activated T cells to help reduce inflammation and tissue damage in patients with moderate-to-severe ulcerative colitis (UC). It will build on a growing body of data generated studying checkpoint agonists in other autoimmune and inflammatory diseases, including rheumatoid arthritis (RA).
Currently there are about 1.5 million people in the U.S. who live with UC, a chronic inflammatory bowel disease (IBD) that impacts the mucosa or lining of the large intestine, or colon, and the rectum. More than 50% of patients with UC feel the disease controls their lives, which is a higher percentage than indicated by patients with asthma, migraines or even RA.[1] And while there are treatment options available, only an estimated one in four treated patients with UC achieve clinical remission after one year of therapy.[2]
Why the PD-1 pathway may be promising for UC
Breaking through the “efficacy ceiling” commonly observed in UC is one of the reasons researchers are studying PD-1 (programmed cell death protein-1) agonist antibodies in this disease. UC, like RA, is considered a T-cell driven autoimmune disease, making it well suited for targeting by monoclonal antibodies directed towards T cells. Studies have shown that at the site of inflamed tissue, as many as 40% of T cells in UC in the lamina propria, and 80% of T cells in RA in the synovium[3], are highly activated and express PD-1 compared to healthy subjects.
In inflammatory diseases such as UC and RA, a person’s immune system is overactive, and the body’s natural built-in “checks and balances” don’t work as they should.[4] Checkpoint agonists, such as PD-1 agonists, target activated T cells in inflamed tissue and the periphery. They work by “hitting the brakes” on runaway inflammation to restore immune balance.
The PD-1 pathway plays an important role in regulating multiple types of immune cells, including two pathogenically distinct T cell subtypes in the context of autoimmune and inflammatory diseases.
The first are T effector cells, which reside primarily in inflamed tissue. T effector cells are referred to as PD-1high when they express high levels of PD-1. These cells secrete inflammatory cytokines, cause tissue damage and perpetuate the inflammatory cycle.
The other distinct types of cells are T follicular helper (Tfh) and T peripheral helper (Tph) cells, which also express high levels of PD-1 and are found primarily in the lymph nodes, spleen and inflamed tissue. If unchecked, these cells drive the immune system response, worsening inflammation and causing tissue damage.[5]
PD-1high T cells are the most pathogenic T cells and represent promising targets to return the composition of the immune system to a less activated state.
PD-1 agonists hold promise for treating UC and RA
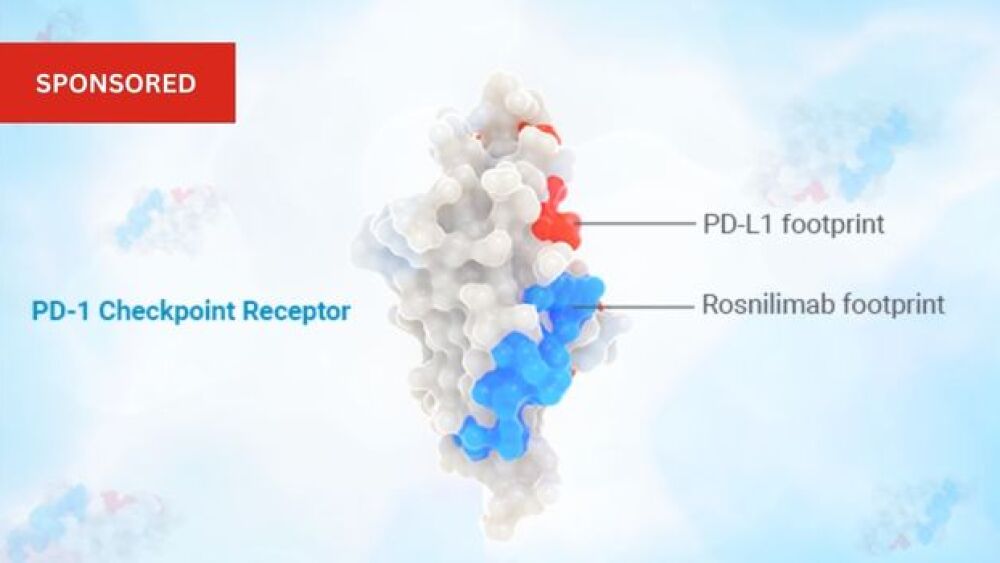
AnaptysBio is studying rosnilimab, which targets specifically PD-1+ T cells, broadly impacting the pathogenic drivers of UC and RA. While many of today’s approved RA and UC treatments generally target one mechanism to fight disease, this PD-1 agonist impacts an essential upstream mediator of inflammation, allowing for the targeting of multiple mechanisms simultaneously.
Anaptys’ monoclonal antibody is engineered to have a unique membrane-proximal binding footprint on PD-1 to bring immune cells tightly together which enables potent depletion and agonism activity.[6]
Rosnilimab has the potential to reduce inflammation and tissue damage, and potentially break through the efficacy ceiling that has limited treatment responses for people living with moderate-to-severe UC and RA.
In a Phase 1 trial in healthy volunteers, rosnilimab was well tolerated with no significant safety signals while demonstrating potent and sustained reduction in peripheral PD-1+ T cells for >30 days. A favorable pharmacokinetic profile was observed, with an estimated two-week half-life for subcutaneous and intravenous routes of administration.
Anaptys anticipates announcing top-line data from its global Phase 2b trial in RA in mid-2025 and top-line data from its global Phase 2 trial in UC in the first half of 2026.
To find out more, visit here.
[1] Rubin DT, et al Dig Dis Sci 2010 [2] Rutgeerts P, et al. N Engl J Med 2005;353:2462–77
[3] Guo Y, Walsh AM, Canavan M, et al. Immune checkpoint inhibitor PD-1 pathway is down-regulated in synovium at various stages of rheumatoid arthritis disease progression. PLOS ONE. 2018. doi.org/10.1371/journal.pone.0192704https://doi.org/10.1371/journal.pone.0192704
[4] Parkin J, Cohen B. An overview of the immune system. Lancet. 2001; 357(9270): 1777-1789. doi:10.1016/S0140-6736(00)04904-7
[5] Akiyama et al, Ann Rheum Dis, 2023
[6] See data in AnaptysBio PD-1 Day presentation, Oct 25, 2023
Sponsored content is written and provided to BioSpace by the advertiser. It is published with the advertiser’s approval without contribution from BioSpace’s editorial and insights teams.