AMSTERDAM, Oct. 13, 2014 /PRNewswire/ -- AbbVie (NYSE: ABBV) announced new results from PIONEER II, a pivotal Phase 3 study, demonstrating the effect of HUMIRA® (adalimumab) in reducing common clinical signs and symptoms in moderate-to-severe hidradenitis suppurativa (HS), specifically the number of abscesses and inflammatory nodules.4 Data were presented as a late-breaking abstract at the 23rd Congress of the European Academy of Dermatology and Venereology (EADV) meeting in Amsterdam. The results of this study, in combination with previously presented results from PIONEER I, will contribute to worldwide regulatory filings for an expanded use of HUMIRA. HUMIRA is not currently approved by regulatory authorities for the treatment of HS.
To view the multimedia assets associated with this release, please click
http://www.multivu.com/players/English/7298751-abbvie-phase-3-hidradenitis-suppurativa-trial-results/

Results from the PIONEER II study found that at 12 weeks, patients with moderate-to-severe HS treated with HUMIRA 40 mg weekly, beginning at week 4 (after HUMIRA 160 mg at week 0 and 80 mg at week 2), achieved a statistically significant greater response compared to those on placebo (58.9 percent versus 27.6 percent, p<0.001).4 Response was defined as an improvement of HS related abscesses and inflammatory nodules at 12 weeks using the Hidradenitis Suppurativa Clinical Response (HiSCR) measure. This is defined as at least 50 percent reduction from baseline in total abscess and inflammatory nodule (AN) count with no increase for either abscess or draining fistula count.5
"HS is painful due to abscesses and nodules, yet there are few treatment options available to help reduce symptoms," said Gregor Jemec, M.D., PIONEER II Study Investigator and Professor of Dermatology at University of Copenhagen, Roskilde Hospital. "Results from the PIONEER trials support the potential for adalimumab to be an important new treatment option for patients with HS."
HS, sometimes referred to as "acne inversa" by dermatologists, is a chronic skin disease characterized by inflamed areas typically located around the armpits, groin, on the buttocks and under the breasts. A number of physical symptoms are associated with HS - namely, nodules and/or abscesses, sinus tracts and scarring. These symptoms make it a painful disease and impact the lives of patients with HS.2,3,6 HS is estimated to affect 1 percent of the general adult population and can be challenging to diagnose as many patients experience a lengthy delay in diagnosis and treatment.1,2 There is currently no cure for HS and there are no approved medications for the treatment of the disease.6
The PIONEER II study is the second pivotal registration study to evaluate the use of HUMIRA in patients with moderate-to-severe HS. Results from PIONEER I also found that moderate-to-severe HS patients treated with HUMIRA 40 mg weekly beginning at week 4 (after 160 mg at week 0 and 80 mg at week 2) achieved a significantly greater response (as measured with HiSCR) compared to placebo at week 12 (41.8 percent versus 26 percent, p = 0.003).4,7
"AbbVie is committed to developing new treatment strategies as part of our focus to helping patients with the most pressing health issues, such as HS," said John Medich, Ph.D., vice president, Clinical Development, Immunology, AbbVie. "We are encouraged by these positive results from the PIONEER trials, which add to the wealth of HUMIRA's clinical trial experience over the past 17 years."
About the Study
PIONEER II is a Phase 3, 36-week, multicenter, randomized, double-blind, two-period study in moderate-to-severe HS patients (n=326). In the first 12-week study period (known as Period A), patients were randomized to receive HUMIRA 160 mg at week 0, 80 mg at week 2 and 40 mg once weekly (n = 163) starting at week 4, or placebo (n = 163). Following Period A, patients were eligible to enroll in a 24-week treatment period (known as Period B). In Period B, patients originally randomized to HUMIRA were re-randomized to receive HUMIRA 40 mg weekly, 40 mg every other week, or placebo. Patients randomized to placebo in Period A remained on placebo in Period B. The primary endpoint was the percentage of patients achieving significant response in improvement of HS severity at 12 weeks using the HISCR measure. The results for Period B have not been presented.4
The most common adverse events (AEs) (>10 percent of subjects in any treatment group) observed in PIONEER II with HUMIRA versus placebo were headache (12.9 percent versus 12.9 percent) and exacerbation of HS (4.3 percent versus 12.9 percent). Serious AEs in patients receiving HUMIRA included infection unknown source (n=1) and acute renal failure (n=1).4
More information on PIONEER I and PIONEER II is available at www.clinicaltrials.gov (NCT01468207 and NCT01468233, respectively).
HUMIRA Therapeutic Indications
Rheumatoid arthritis
HUMIRA in combination with methotrexate is indicated for:
- the treatment of moderate to severe, active rheumatoid arthritis in adult patients when the response to disease-modifying anti-rheumatic drugs including methotrexate has been inadequate.
- the treatment of severe, active and progressive rheumatoid arthritis in adults not previously treated with methotrexate.
HUMIRA has been shown to reduce the rate of progression of joint damage as measured by X-ray and to improve physical function, when given in combination with methotrexate.
Polyarticular juvenile idiopathic arthritis
HUMIRA in combination with methotrexate is indicated for the treatment of active polyarticular juvenile idiopathic arthritis, in children and adolescents from the age of 2 years who have had an inadequate response to one or more disease-modifying anti-rheumatic drugs (DMARDs). HUMIRA has not been studied in children aged less than 2 years.
Enthesitis-related arthritis
HUMIRA is indicated for the treatment of active enthesitis-related arthritis in patients, 6 years of age and older, who have had an inadequate response to, or who are intolerant of, conventional therapy.
Ankylosing spondylitis (AS)
HUMIRA is indicated for the treatment of adults with severe active ankylosing spondylitis who have had an inadequate response to conventional therapy.
Axial spondyloarthritis without radiographic evidence of AS
HUMIRA is indicated for the treatment of adults with severe axial spondyloarthritis without radiographic evidence of AS but with objective signs of inflammation by elevated CRP and / or MRI, who have had an inadequate response to, or are intolerant to nonsteroidal anti-inflammatory drugs.
Psoriatic arthritis
HUMIRA is indicated for the treatment of active and progressive psoriatic arthritis in adults when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate. HUMIRA has been shown to reduce the rate of progression of peripheral joint damage as measured by X-ray in patients with polyarticular symmetrical subtypes of the disease and to improve physical function.
Psoriasis
HUMIRA is indicated for the treatment of moderate to severe chronic plaque psoriasis in adult patients who failed to respond to or who have a contraindication to, or are intolerant to other systemic therapy including cyclosporine, methotrexate or PUVA.
Crohn's disease
HUMIRA is indicated for treatment of moderately to severely active Crohn's disease, in adult patients who have not responded despite a full and adequate course of therapy with a corticosteroid and/or an immunosuppressant; or who are intolerant to or have medical contraindications for such therapies.
Pediatric Crohn's disease
HUMIRA is indicated for the treatment of severe active Crohn's disease in pediatric patients (from 6 years of age) who have had an inadequate response to conventional therapy including primary nutrition therapy, a corticosteroid, and an immunomodulator, or who are intolerant to or have contraindications for such therapies.
Ulcerative colitis
HUMIRA is indicated for treatment of moderately to severely active ulcerative colitis in adult patients who have had an inadequate response to conventional therapy including corticosteroids and 6-mercaptopurine (6-MP) or azathioprine (AZA), or who are intolerant to or have medical contraindications for such therapies.
Important Safety Information
HUMIRA is a human monoclonal antibody that binds to tumor necrosis factor-alpha (TNF-). People should not use HUMIRA if they are allergic to adalimumab or any other ingredients of HUMIRA, have a severe infection including active tuberculosis (TB), or have moderate to severe heart failure.
People may get infections more easily while using HUMIRA. This risk may increase if lung function is impaired. These infections may be serious and include TB; infections caused by viruses, fungi, parasites or bacteria, or other opportunistic infections and sepsis that may, in rare cases, be life-threatening. Cases of TB have been reported in people treated with HUMIRA. People should be checked for both active or inactive TB and hepatitis B virus (HBV) before starting HUMIRA and be monitored for signs and symptoms during and after therapy. People should tell their doctor if they live in or have traveled to a region where fungal infections are common, have a history of recurrent infections; or have an increased risk of getting infections. People over 65 years of age may be more susceptible to infections while taking HUMIRA.
There have been cases of certain kinds of cancer in people taking HUMIRA or other TNF blockers. The risk of getting lymphoma, leukemia, or other cancers may increase. On rare occasions, a severe type of cancer called hepatosplenic T-cell lymphoma has been observed and often results in death. In addition, cases of non-melanoma skin cancer have been observed in patients taking HUMIRA.
Other possible serious side effects with HUMIRA include reactivation of hepatitis B in chronic carriers of this virus, central nervous system problems, allergic reactions including anaphylaxis, blood problems, new or worsening congestive heart failure, and certain immune reactions such as a lupus-like syndrome.
Certain vaccines may cause infections and should not be given while receiving HUMIRA. HUMIRA may have a minor influence on the ability to drive and use machines. The use of HUMIRA with anakinra or abatacept is not recommended.
The most commonly reported adverse reactions include injection site reactions (including pain, swelling, redness, or itching), respiratory tract infections (including a cold, runny nose, sinus infection, or pneumonia), headache, abdominal pain, nausea, vomiting, rash, and musculoskeletal pain.
HUMIRA is given by injection under the skin. The benefits and risks of HUMIRA should be carefully considered before starting therapy.
Globally, prescribing information varies; refer to the individual country product label for complete information.
About AbbVie
AbbVie is a global, research-based biopharmaceutical company formed in 2013 following separation from Abbott Laboratories. The company's mission is to use its expertise, dedicated people and unique approach to innovation to develop and market advanced therapies that address some of the world's most complex and serious diseases. AbbVie employs approximately 25,000 people worldwide and markets medicines in more than 170 countries. For further information on the company and its people, portfolio and commitments, please visit www.abbvie.com. Follow @abbvie on Twitter or view careers on our Facebook or LinkedIn page.
Forward-Looking Statements
Some statements in this news release may be forward-looking statements for purposes of the Private Securities Litigation Reform Act of 1995. The words "believe," "expect," "anticipate," "project" and similar expressions, among others, generally identify forward-looking statements. AbbVie cautions that these forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those indicated in the forward-looking statements. Such risks and uncertainties include, but are not limited to, challenges to intellectual property, competition from other products, difficulties inherent in the research and development process, adverse litigation or government action, and changes to laws and regulations applicable to our industry.
Additional information about the economic, competitive, governmental, technological and other factors that may affect AbbVie's operations is set forth in Item 1A, "Risk Factors," in AbbVie's 2013 Annual Report on Form 10-K, which has been filed with the Securities and Exchange Commission.
AbbVie undertakes no obligation to release publicly any revisions to forward-looking statements as a result of subsequent events or developments, except as required by law.
References
- Dufour DN, Emtestam L, Jemec GB. Hidradenitis Suppurativa: A Common and Burdensome, Yet Under-Recognised, Inflammatory Skin Disease. Postgrad Med J. 2014; 90 (1062):216-21.
- Jemec G. Hidradenitis Suppurativa. N Engl J Med. 2012; 366:158-64.
- Zouboulis CC, Tsatsou F (2012) Disorders of the Apocrine Sweat Glands. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K (eds) Fitzpatrick's Dermatology in General Medicine. 8th ed, McGraw Hill, New York Chicago, pp 947-959.
- Efficacy and Safety of Adalimumab in Patients with Moderate to Severe Hidradenitis Suppurativa: Results from PIONEER II, a Phase 3, Randomized, Placebo-Controlled Trial. Abstract FC08.2. 22nd Congress of the European Dermatology and Venereology (EADV) Meeting, Amsterdam, Netherlands 2014.
- Kimball, AB, Jemec, GB. Assessing the Validity, Responsiveness, and Meaningfulness of the Hidradenitis Suppurativa Clinical response (HiSCR) as the Clinical Endpoint for Hidradenitis Suppurativa Treatment. Br J Dermatol. 2014.
- Mayo Health Clinic. Hidradenitis Suppurativa. Available at: http://www.mayoclinic.com/health/hidradenitis-suppurativa/DS00818. Published April 9, 2013. Accessed September 10, 2013.
- Safety and Efficacy of Adalimumab in Patients with Moderate to Severe Hidradenitis Suppurativa: Results from First 12 Weeks of PIONEER I, a Phase 3, Randomized, Placebo-Controlled Trial. Abstract #177 and 210. 44th Annual Meeting of the European Society for Dermatological Research (ESDR), Copenhagen, Denmark 2014. http://www.nature.com/jid/journal/v134/n2s/full/jid2014340a.html
- American Academy of Dermatology. Hidradenitis Suppurativa. Available at: http://www.aad.org/dermatology-a-to-z/diseases-and-treatments/e---h/hidradenitis-suppurativa. Accessed August 28, 2014.
U.S. Media: | Investors: |
Alissa Bolton | Liz Shea |
+1 (847) 937-2644 | +1 (847) 935-2211 |
International Media: | |
Kelli Teno | |
+1 (847) 935-9593 |

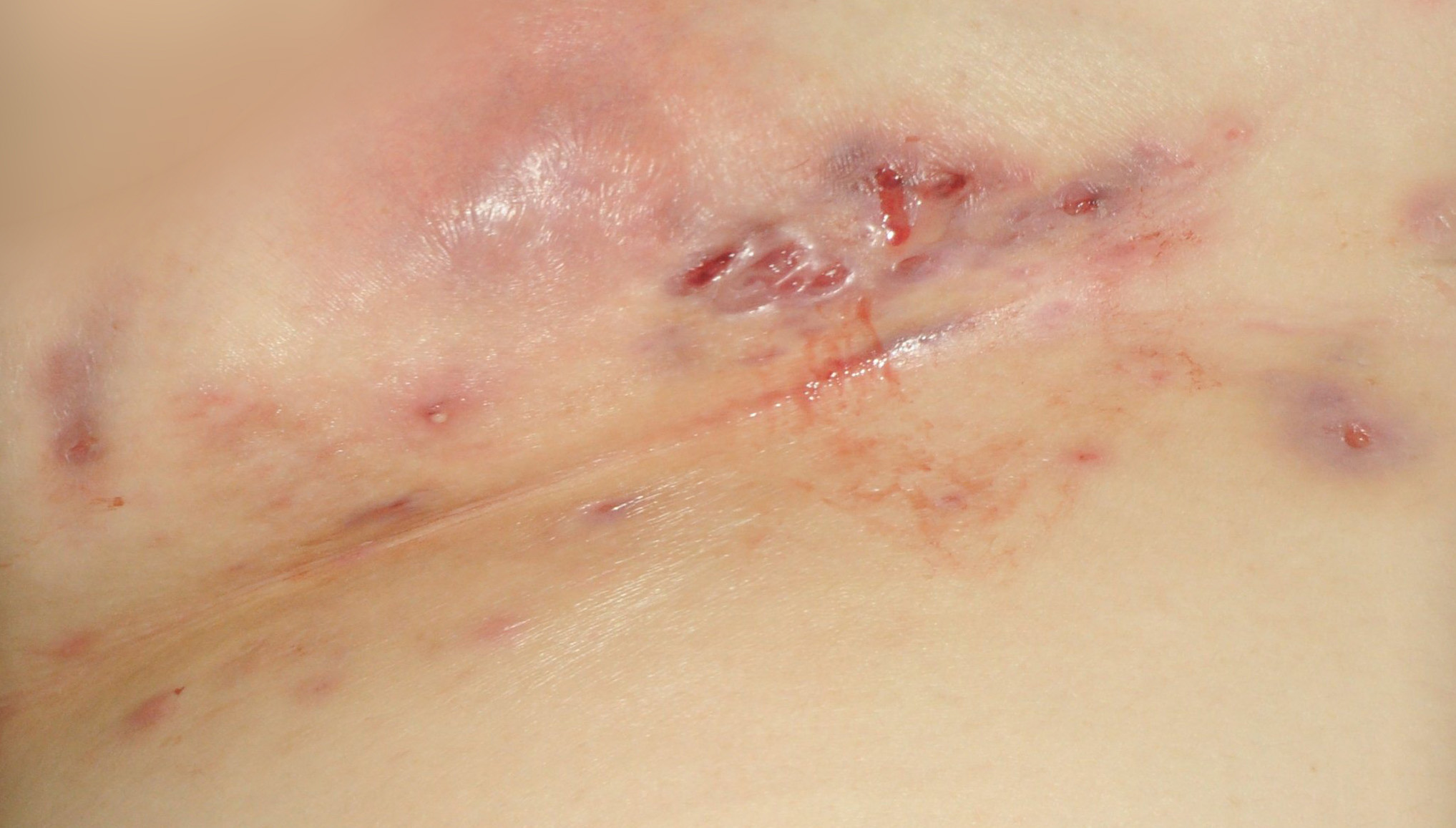
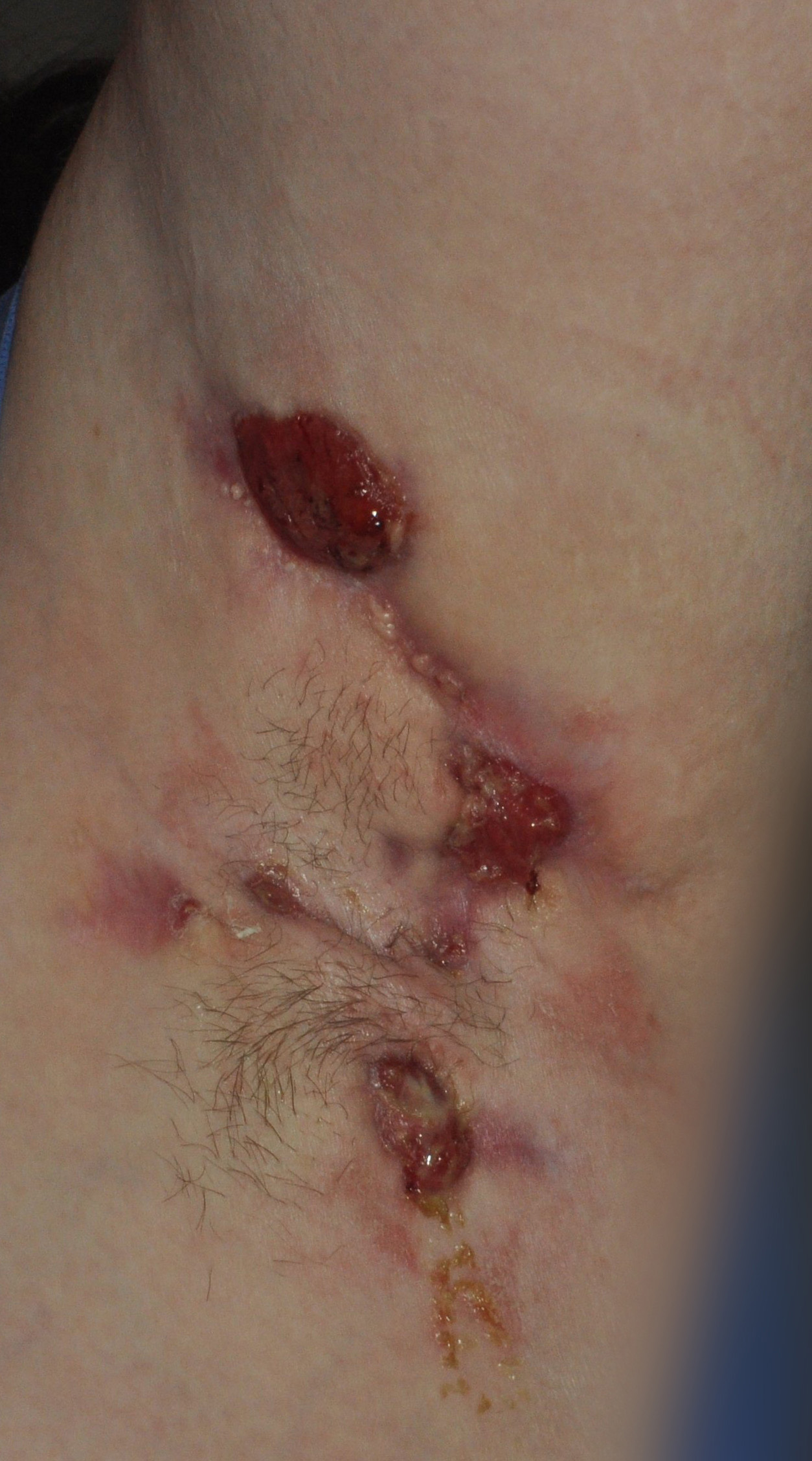
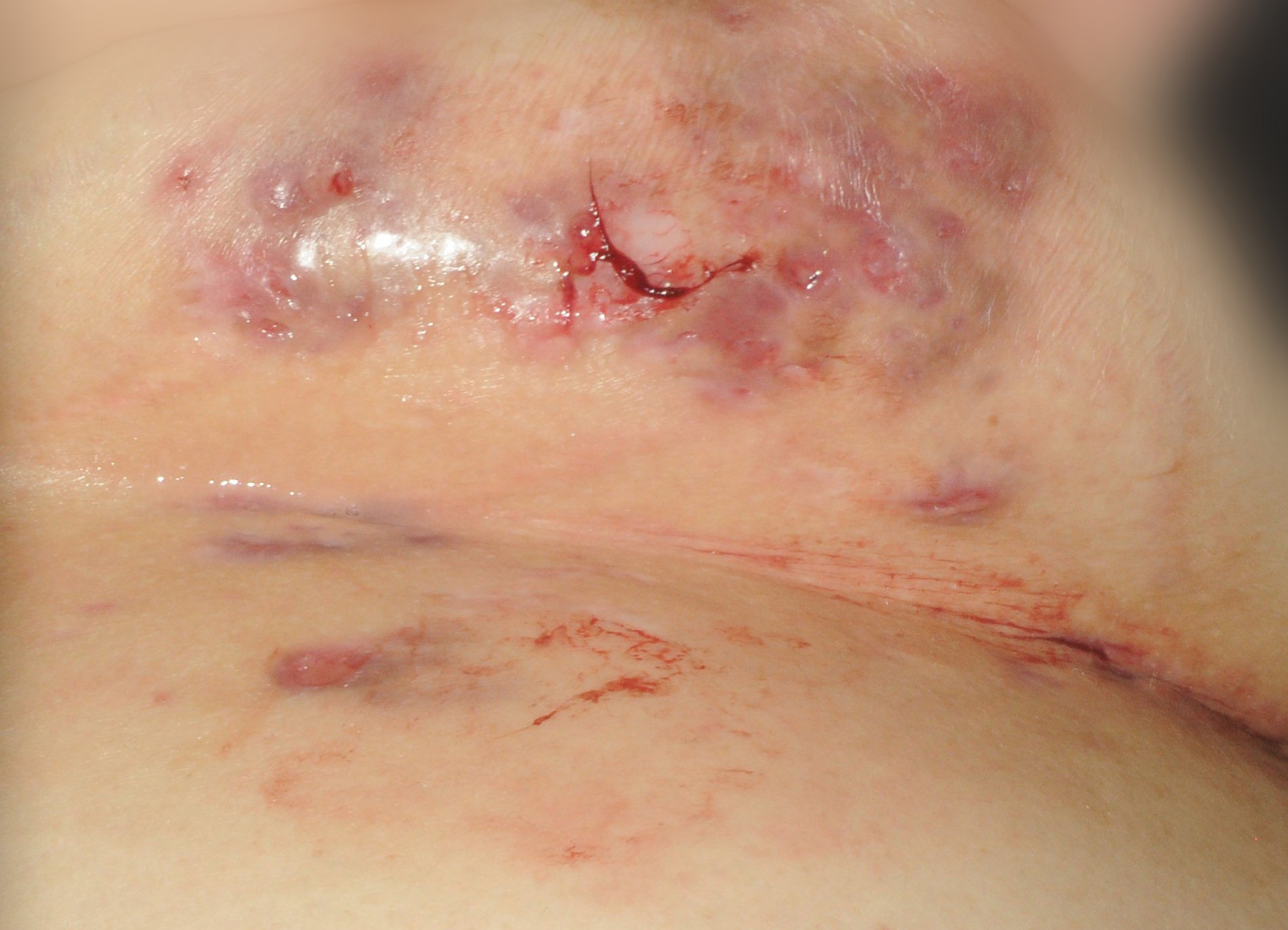
To view the multimedia assets associated with this release, please click
http://www.multivu.com/players/English/7298751-abbvie-phase-3-hidradenitis-suppurativa-trial-results/
SOURCE AbbVie




