ReGelTec, Inc., a medical device company developing a percutaneous treatment for chronic low back pain, announced that eleven patients with degenerative disc disease have been enrolled in the Company’s Early Feasibility Study in Barranquilla, Colombia.

The company also secures more than $3.75M in Series A Funding
BALTIMORE--(BUSINESS WIRE)-- ReGelTec, Inc., a medical device company developing a percutaneous treatment for chronic low back pain, announced today that eleven patients with degenerative disc disease have been enrolled in the Company’s Early Feasibility Study in Barranquilla, Colombia. The procedures were proctored remotely via Zoom and all eleven patients were successfully treated with HYDRAFIL™, a patented hydrogel that is melted prior to injection into the nucleus of a degenerated disc via a 17-gauge needle. When HYDRAFIL™ cools to body temperature it forms a contiguous implant within the nucleus of the degenerated disc to augment the residual nucleus pulposus, restore normal biomechanical properties of the disc and alleviate pain. The procedures were completed while patients were awake and under local anesthesia in an outpatient clinic. Patients were up and walking within one to two hours of the injection. Most patients were sent home on standard over the counter pain medication and procedural related pain generally resolved within 24-72 hours. Patient follow-up is ongoing with initial patients reporting significant pain reduction and improvements in functional status at 30-days.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20201014005342/en/

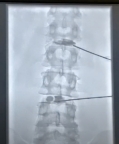
Dual level HYDRAFIL implant procedure at L2/L3 and L4/L5 (Photo: Business Wire)
ReGelTec also announced that the company has secured more than $3.75M in Series A financing during the COVID-19 pandemic. The funds will be used to support continued enrollment in the Early Feasibility Study in Colombia and ongoing development activities required to begin clinical studies in the United States.
“Back pain is the single most common cause of disability worldwide and degenerative disc disease is the leading cause of chronic low back pain,” said Douglas Beall, MD the chairman of the company’s medical advisory board. “There has been a significant unmet clinical need to repair degenerated lumbar discs for decades. Based on the initial clinical results, HYDRAFIL™ appears to be uniquely positioned to treat degenerative disc disease by functionally replacing a substantial amount of disc material lost to disc degeneration. The early results are very promising, and I am very excited to continue working on the HYDRAFIL™ clinical program.”
Bill Niland, a serial entrepreneur and the company’s CEO said “I have three degenerated discs and have had multiple procedures over the last 30 years, so after we sold Harpoon Medical to Edwards Lifesciences the opportunity to develop a technology for a condition that affect me personally was very appealing. I had a team of talented medical device executives who were excited for their next project and Dr. Lowman, the lead inventor, had a technology that was ready for the clinic. The COVID-19 pandemic has added complexity and additional challenges to conducting the early clinical work, which makes our clinical results even more exciting.”
ABOUT REGELTEC, INC:
ReGelTec, Inc. is a clinical stage medical device company commercializing HYDRAFIL™, a percutaneous treatment for low back pain due to degenerative disc disease. The company was formed when a team of chemical engineers with extensive experience in polymer science partnered with a cross-functional team of medical device professionals who had recently sold their former company Harpoon Medical, Inc. to Edwards Lifesciences. Once approved, the HYDRAFIL™ System will offer patients suffering from chronic back pain due to degenerative disc disease a non-surgical treatment option when traditional conservative care fails.
View source version on businesswire.com: https://www.businesswire.com/news/home/20201014005342/en/
Bill Niland, CEO
Phone: (443) 956-4465
bniland@regeltec.com
Source: ReGelTec, Inc.
View this news release and multimedia online at:
http://www.businesswire.com/news/home/20201014005342/en