News
Novartis will still be on the lookout for early-stage deals under $2 billion, and later-stage agreements around a product that could reach the market within five years, CEO Vas Narasimhan said Wednesday.
FEATURED STORIES
After advancing in lockstep through the pandemic, the fortunes of the biotechs have diverged as their use of COVID-19 windfalls has taken shape.
After suffering in the wake of expired tax incentives for pharmas, the island is trying to take advantage of geopolitics to grow its drug manufacturing sector.
AstraZeneca’s $15 billion pledge to its China operations highlights the country’s advantages. But other regions are also hoping to host more clinical studies.
FROM OUR EDITORS
Read our takes on the biggest stories happening in the industry.
Phacilitate’s annual event dawns as cell and gene therapies reach a new tipping point: the science has hit new heights just as regulatory and government policies spark momentum and frustration.
THE LATEST
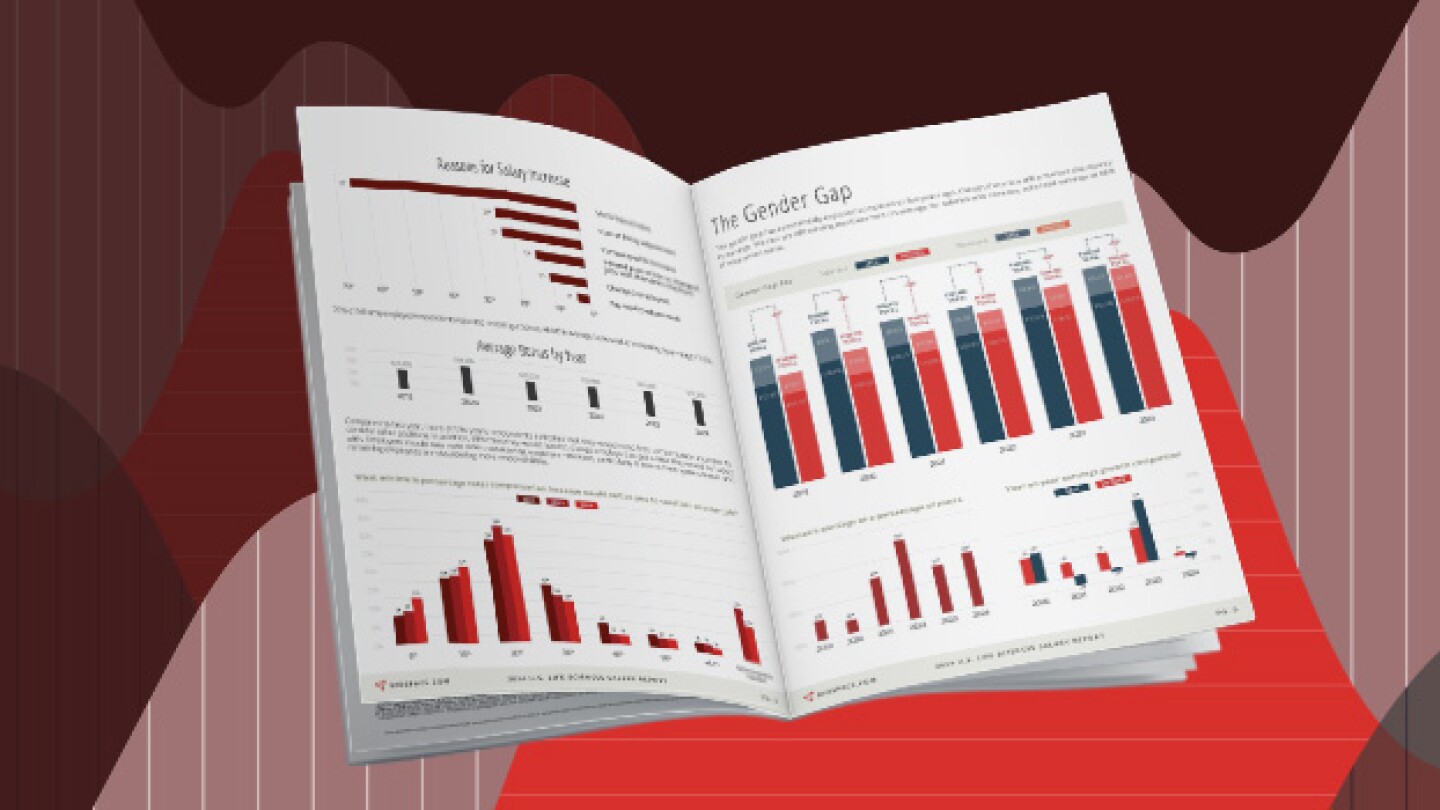
BioSpace’s 2024 Salary Report explores the average salaries and salary trends of life sciences professionals.
AstraZeneca reported Monday that adding Lynparza to Imfinzi improved outcomes in mismatch repair proficient endometrial cancer, more than doubling the median duration of response in patients.
With Boehringer Ingelheim’s announcement earlier this month that it was capping U.S. inhaler costs at $35 per month, AstraZeneca on Monday followed suit.
New late-stage trial results for GSK’s Jemperli show improved overall and progression-free survival in a broader range of endometrial cancer patients, which could lead to a potential label expansion.
Contineum Therapeutics joined the 2024 initial public offering class on Friday with an SEC filing. The biotech will use the IPO proceeds to complete a Phase II trial for its most mature candidate targeting multiple sclerosis.
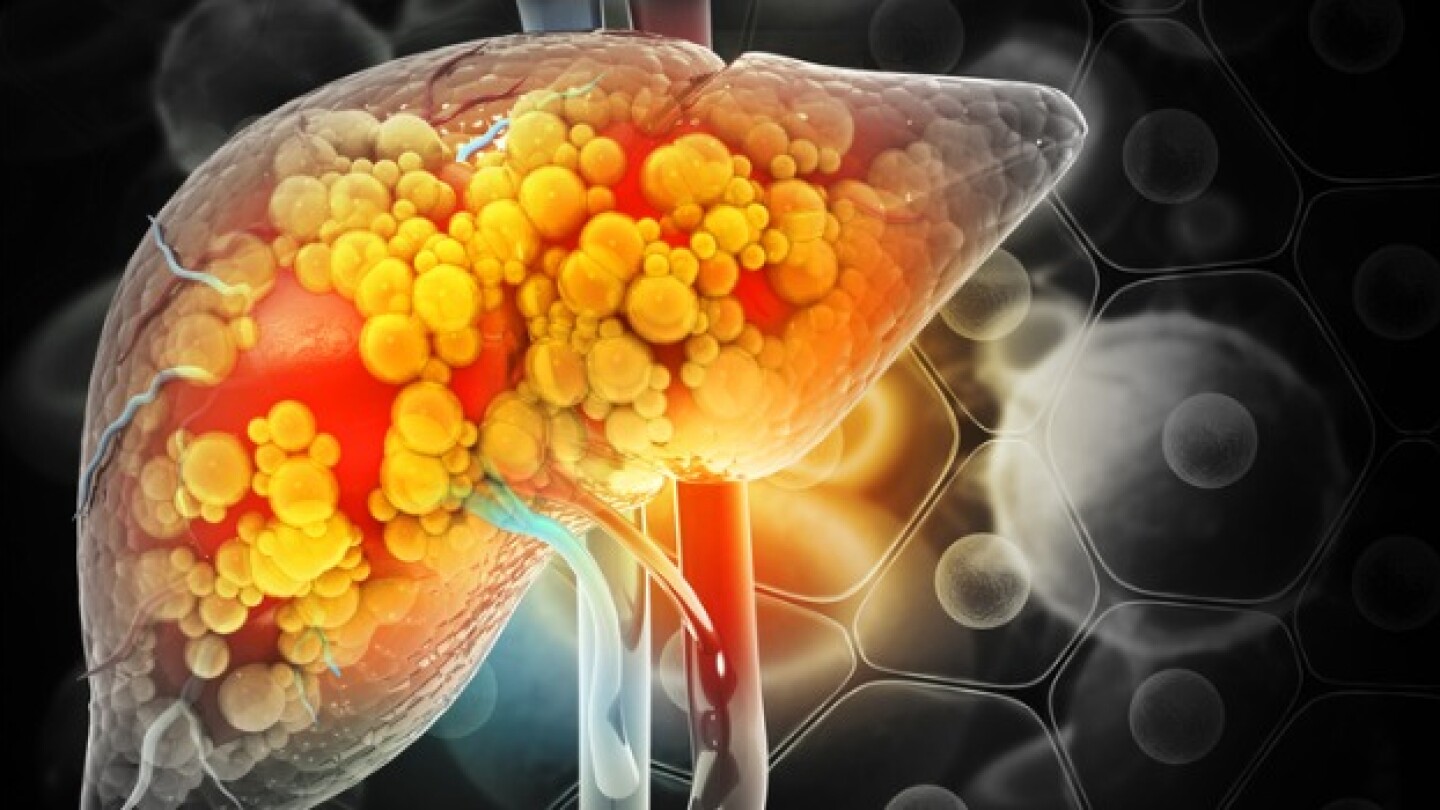
With its FDA approval last week and first-to-market advantage, Madrigal Pharmaceuticals’ Rezdiffra will set the standard for other metabolic dysfunction-associated steatohepatitis candidates in development.
By votes of 11-0 and 8-3, respectively, an FDA advisory committee Friday deemed the risks of early death for both Johnson & Johnson’s Carvykti and Bristol Myers Squibb’s Abecma acceptable.
Asgard Therapeutics, a Swedish gene therapy biotech, has closed a $32 million Series A round with help from prominent pharma players as it prepares for a 2026 IND.
Bayer will co-create a novel target identification platform that leverages Aignostics’ artificial intelligence technology and proprietary multimodal patient cohorts.
The Chinese biotechs are broadening their collaboration. Hansoh Pharma is licensing Biotheus’ anti-EGFR/cMet bispecific antibody to develop antibody-drug conjugates.