News
FDA Commissioner Marty Makary last week announced a directive that would limit industry participation in the agency’s advisory committees. But not only do company reps serve only as non-voting members, a 1997 law actually requires industry involvement.
FEATURED STORIES
On June 10, the FDA will convene its Peripheral and Central Nervous System Drugs Advisory Committee to discuss the New Drug Application for Lilly’s Alzheimer’s drug.
The Celularity CEO and founder tells BioSpace he believes that placenta-derived cells are the future of stem cell therapies to fight autoimmune disease, cancer, even aging.
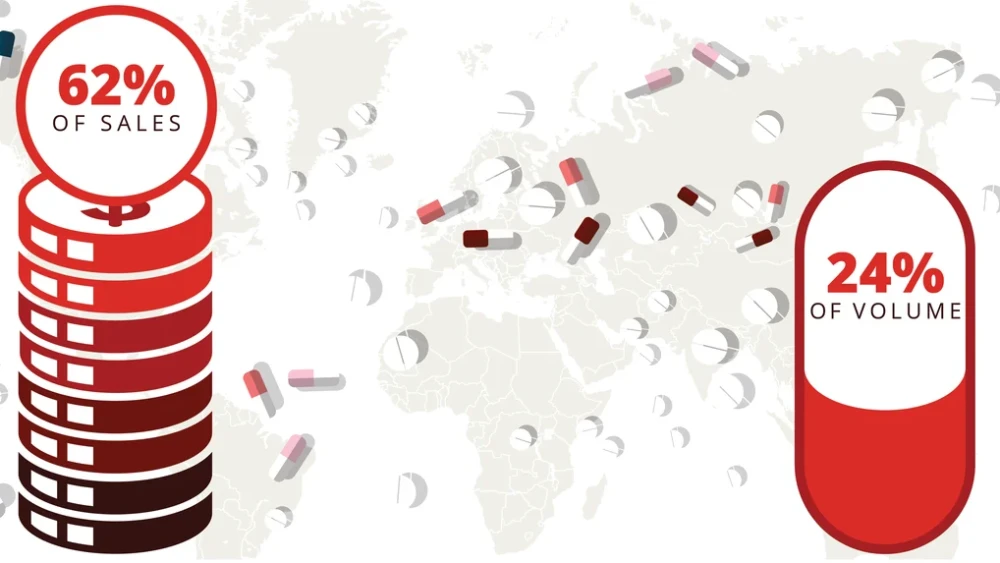
In this deep dive, BioSpace takes a closer look at the drug price crisis in the U.S. As President Joe Biden and former President Donald Trump gear up for a rematch in the 2024 election, we explore how federal reforms to lower costs could be leveraged on the campaign trail.
Job Trends
The Center for Disease Analysis Foundation announced that it received an $8 million grant from Gilead Sciences as part of Gilead’s Relink grant program.
FROM OUR EDITORS
Read our takes on the biggest stories happening in the industry.
Unpredictable communication and a lack of transparency are eroding the industry’s and the public’s trust. The FDA, experts agree, needs to take control of the narrative.
THE LATEST
The data suggest the high dose nearly closes the efficacy gap with Zepbound.
Biopharma executives make their predictions for the year ahead, from a bold forecast for the return of the megadeal to a plea for the slow, healthy recovery of the industry at large.
Drugmakers will have until the end of February to decide whether they want to participate in the second round of Medicare negotiations or not. CMS has until June 1 to send an initial offer for the adjusted prices.
The Phase III CodeBreaK 300 study returned disappointing overall survival data for Lumakras plus Vectibix in metastatic colorectal cancer, but in its approval announcement, the FDA pointed to significant improvements in progression-free survival, calling it the “major efficacy outcome” of the trial.
Novartis is locked in a legal back-and-forth with MSN Pharma over alleged patent infringement of its heart failure drug Entresto.
Boehringer Ingelheim’s trio of late-stage schizophrenia failures on Thursday came a day after the Department of Health and Human Services hit back on the pharma’s legal challenge to the IRA’s drug price negotiation program.
As I ran from interview to interview across San Francisco, I was consistently warmed by the stories I was told by biotech and pharma executives—and the general comradery in the air throughout the chaotic event.
Atara Therapeutics’ Ebvallo, already marketed in Europe for a transplant-related blood cancer, will not hit the U.S. market just yet, forcing the company to “significantly reduce expenses.”
With incoming president Donald Trump threatening a trade war with China, experts told BioSpace that the new administration will likely understand why medicines should be treated differently.
In this episode of Denatured, BioSpace’s Head of Insights Lori Ellis talks to Dr. Peter Marks, Director, CBER about his thoughts on the future of cell and gene therapies.