News
Moderna will not commit to previous 2028 breakeven guidance as the ripple effects of the FDA’s refusal-to-file decision spread through its pipeline.
FEATURED STORIES
The rare disease drugmaker is facing potential competitors for achondroplasia drug Voxzogo. Is a big M&A deal with two approved assets enough to maintain investor interest?
The FDA issued a rare Refusal-to-File letter to Moderna over its mRNA-based influenza vaccine application, in an unusual move that sent the biotech’s shares tumbling.
A rapturous response to data published last year for Pelage’s hair loss candidate overwhelmed the biotech. Now, the company is ready to show the world the science behind the breakthrough.
FROM OUR EDITORS
Read our takes on the biggest stories happening in the industry.
Novo Nordisk and Eli Lilly have been battling head-to-head in an exploding obesity market. They should never have been compared apples to apples.
THE LATEST
The FDA approved Bristol Myers Squibb’s Breyanzi for chronic lymphocytic leukemia and small lymphocytic leukemia prior to Friday’s adcomm for the company’s other CAR-T therapy, Abecma.
Despite skepticism from FDA reviewers, the Oncologic Drugs Advisory Committee on Thursday strongly supported Geron’s imetelstat for the treatment of anemia in patients with lower-risk myelodysplastic syndromes.
After several delays, BeiGene on Thursday finally secured the FDA’s approval for its PD-1 inhibitor Tevimbra for the treatment of unresectable or metastatic esophageal squamous cell carcinoma.
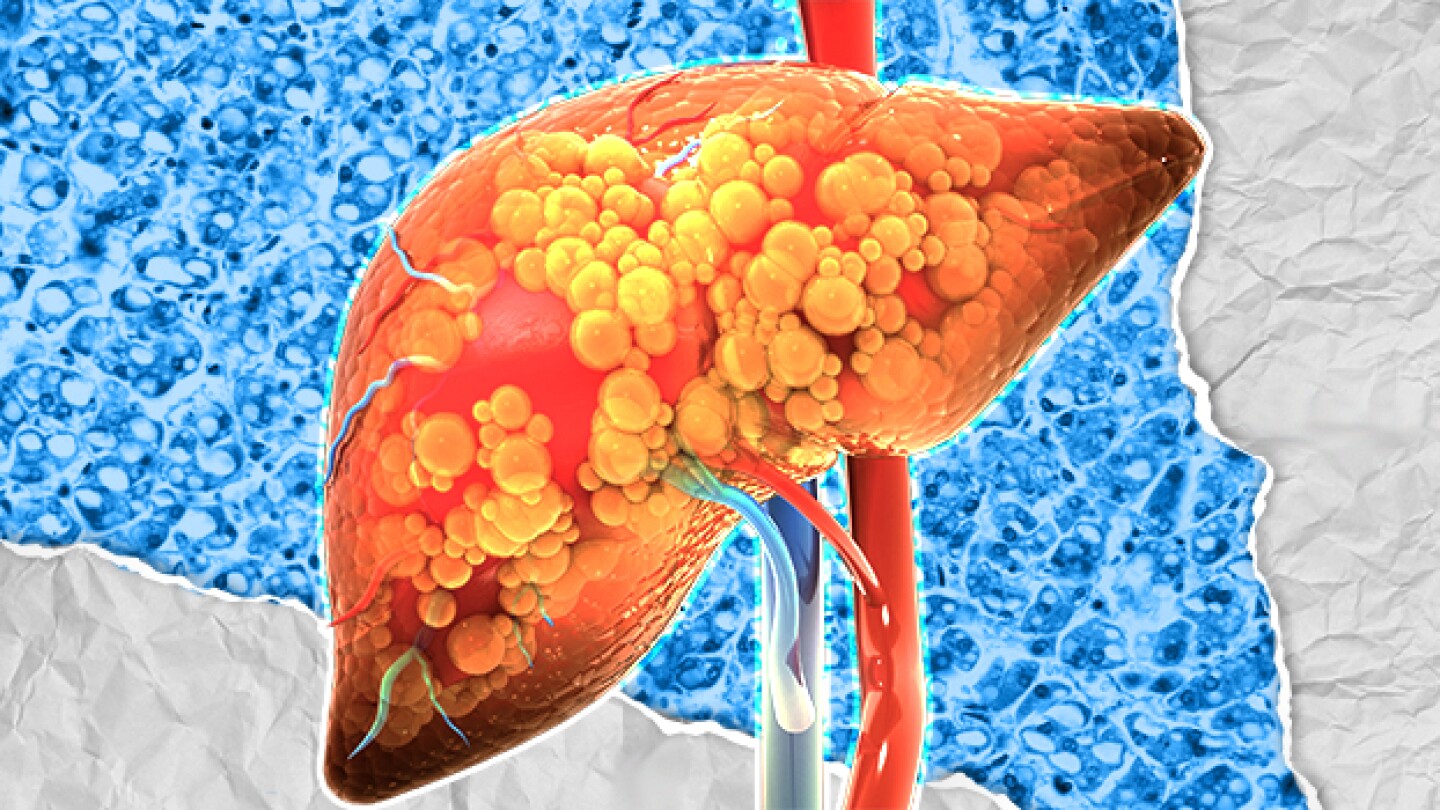
If you’re confused by the NASH versus MASH indication, you’re not alone.
Madrigal Pharmaceuticals’ Rezdiffra (resmetirom) is the first-ever approved therapy for metabolic dysfunction-associated steatohepatitis—a decision experts say could signal a sea change in treatment of the disease.
The U.K. National Institute for Health and Care Excellence on Thursday recommended against funding Vertex Pharmaceuticals’ CRISPR-based sickle cell disease therapy Casgevy unless uncertainties can be cleared up.
This episode focuses on a healthy discussion regarding the IRA, particularly the unintended consequences to small molecule development within the industry and for patients.
At the center of the deal is Amolyt Pharma’s late-stage candidate eneboparatide for the rare disease hypoparathyroidism. AstraZeneca also gains ownership of AZP-3813, which is being assessed for acromegaly in a Phase I trial.
A lawsuit filed by the Pharmaceutical Research and Manufacturers of America failed to block an Arkansas law that empowers hospitals to use outside pharmacies to dispense discounted drugs.
Wednesday’s FDA approval expands Mirum’s Livmarli into the rare genetic disorder that causes progressive liver disease. The biotech has also filed a supplemental New Drug Application for a higher dose of the drug and allowing its use in younger patients.