Mirimus, Inc., a leader in conducting high-volume, high-quality PCR testing, today announced it has been named as one of five winners in XPRIZE Rapid COVID Testing for its pioneering SalivaClear™ COVID-19 pooled saliva testing platform.
|
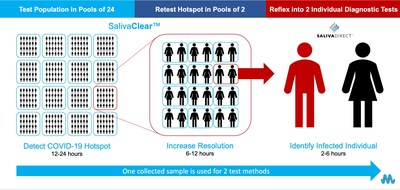
NEW YORK, March 16, 2021 /PRNewswire/ -- Mirimus, Inc., a leader in conducting high-volume, high-quality PCR testing, today announced it has been named as one of five winners in XPRIZE Rapid COVID Testing for its pioneering SalivaClear™ COVID-19 pooled saliva testing platform. The SalivaClear platform is composed of three key elements – saliva-based sampling, pooled testing and gold-standard PCR molecular diagnostics – that, when combined, enable frequent, high-quality, high-throughput, low-cost detection of SARS-CoV-2, the virus that causes COVID-19. XPRIZE Rapid Covid Testing is a $6 million, six-month competition to develop faster, cheaper, and easier to use COVID-19 testing methods at scale. As one of five winners in the main track of the competition, Mirimus was awarded $500,000 and has the opportunity to receive a total XPRIZE award of $1 million. The winning teams will now advance to the deployment phase of the competition where each company will prepare and administer tests in collaboration with OpenCovidScreen, a non-profit founded by scientists and business leaders to drive needed innovation through "Open Science." Each team that successfully completes the deployment phase will be rewarded an additional $500,000, for the total $1 million prize. "We are honored to have been named a winner in the XPRIZE Rapid COVID Testing competition and are also very appreciative of XPRIZE for enabling us to greatly accelerate our scalability. We have now increased our volume to 100,000 tests per week and are working to scale to 1 million through our partner lab network over the next few months," said Prem Premsrirut, M.D., Ph.D., Co-founder and CEO of Mirimus. "SalivaClear is a highly effective, efficient and cost-effective solution to COVID-19 surveillance testing that has been successfully utilized by hundreds of schools, businesses and government organizations across the country to conduct surveillance testing of groups of individuals in order to quickly isolate COVID-19 hotspots before they can become outbreaks. Even as the rollout of COVID-19 vaccines continues, robust surveillance testing remains a necessary part of any reopening strategy, especially with the recent emergence of dangerous viral variants." Saliva-based COVID-19 testing with SalivaClear is simple, safe and noninvasive. Unlike intranasal swabs or blood samples, saliva samples can be easily and painlessly self-collected without the need for needles, nose swabs or medical personnel. Pooled testing entails combining a small volume of saliva (200 microliters) from up to 24 individual samples to create a single specimen for analysis. A diagnostic is then performed on the entire pool. If a positive test occurs, the pool is narrowed to pinpoint the positive individual(s). In a recently published scientific paper, entitled, "Pooled Surveillance Testing Program for Asymptomatic SARS-CoV-2 Infections in K-12 Schools and Universities," SalivaClear was shown to substantially reduce the cost associated with PCR testing and allow schools to rapidly assess transmission and make data-driven decisions to adjust prevention protocols. Additionally, the research determined that SalivaClear provides a similar sensitivity to the molecular assay of individual samples, in terms of both qualitative (100% agreement of results on both pooled and individual samples) and quantitative (comparable cycle threshold values between pooled and individual samples) measures. About XPRIZE About SalivaClear About Mirimus, Inc. Media Contacts:
SOURCE Mirimus, Inc. |