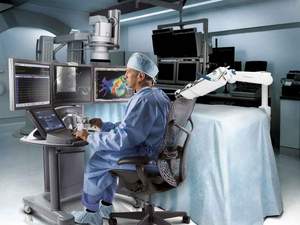
BOSTON, MA--(Marketwire - May 13, 2009) - Booth # 1267 - Hansen Medical, Inc. (NASDAQ: HNSN) will showcase advancements to its Sensei® Robotic Catheter System for electrophysiology (EP) procedures at Heart Rhythm 2009, the Heart Rhythm Society's 30th Annual Scientific Sessions in Boston, May 13 - 16. The company will demonstrate improvements in its user interface, catheter capabilities and design, and in imaging and mapping technology integration.
"It is our goal to continue to offer physicians preeminent technological solutions that provide confidence and accuracy during complex EP procedures," said Fred Moll, president and CEO of Hansen Medical. "We are very encouraged by the broad adoption of our technology worldwide with 65 systems shipped through March 31, 2009 and the significant volume of electrophysiology procedures performed using our flexible robotic platform."
The company will show its new tactile vibration feature for its IntelliSense® Fine Force Technology, which provides physicians with tactile feedback on proximal force for the first time with the Sensei system. As a result, physicians can now feel IntelliSense's force measurements through vibration of the Instinctive Motion Controller (IMC). In addition, physicians will continue to see a visual measure of force on the Sensei system's display monitor during EP procedures. With this new feature, physicians now have an improved tactile understanding of IntelliSense's measurements and force sensing during complex cardiac procedures. This advancement is important because a study suggests a link between the ability to measure force and the quality of electroanatomical maps(1).
"The new vibration feature for IntelliSense is an improvement to the Sensei system because I believe it allows me to concentrate on all the other aspects of the procedure while safely manipulating the catheter," said Laurence M. Epstein, M.D., chief, arrhythmia service, Cardiovascular Division, Brigham and Women's Hospital in Boston. "In addition, I have more confidence in the 3D surface maps I create because I can more easily adjust the force."
Hansen Medical will also showcase its CoHesion™ 3D Visualization Module, an integrated product that combines the 3D visualization of the EnSite™ electroanatomic mapping system from St. Jude Medical, with 3D navigation from the Sensei system. This integrated solution allows physicians the opportunity to navigate the robotic Artisan™ Control Catheter instinctively in St. Jude Medical's electroanatomic map. A European clinical study has demonstrated reductions in both procedure and fluoroscopy time when using the CoHesion product(2).
The Sensei system is built upon an open architecture design, which enables physicians to accurately deliver instinctive, flexible and stable catheter control during complex cardiac arrhythmia procedures. At the same time, physicians can choose and use their own preferred products because of this open architecture. Hansen Medical's commitment to open architecture allows for compatibility with a broad range of leading imaging companies including St. Jude Medical, Philips Healthcare and GE Healthcare. At Heart Rhythm 2009, Philips and Hansen Medical will reveal first results of their collaborative effort to further integrate the Xper Allura EP intervention lab with the Sensei system. Additionally, GE Healthcare will be featuring a Hansen Medical kiosk in the GE Healthcare booth.
Hansen Medical's technology is compatible with fluoroscopy, ultrasound, 3D surface map and patient electroanatomical data. The two main components that comprise the Sensei system are the Artisan catheter and the ergonomically designed, remotely placed workstation. The physician workstation is adaptable to existing EP lab suites and imaging technologies and can be placed away from the field of direct radiation.
About Hansen Medical, Inc.
Hansen Medical, Inc., based in Mountain View, Calif., develops products and technology using robotics for the accurate positioning, manipulation and control of catheters and catheter-based technologies. Its first product, the Sensei Robotic Catheter system, was cleared by the U.S. Food and Drug Administration (FDA) in May 2007 for manipulation and control of certain mapping catheters in electrophysiology (EP) procedures. The safety and effectiveness of the Sensei system for use with cardiac ablation catheters in the treatment of cardiac arrhythmias, including atrial fibrillation (AF), have not been established. The Sensei system is CE Marked for use during EP procedures, such as guiding catheters in the treatment of AF. Additional information can be found at www.hansenmedical.com.
Forward-Looking Statements
This press release contains forward-looking statements regarding, among other things, statements relating to expectations, goals, plans, objectives and future events. Hansen Medical intends such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 21E of the Exchange Act and the Private Securities Litigation Reform Act of 1995. Examples of such statements include statements about the creation of integrated products and the resulting benefits of such products. These statements are based on the current estimates and assumptions of Hansen Medical management as of the date of this press release and are subject to risks, uncertainties, changes in circumstances, assumptions and other factors that may cause actual results to differ materially from those indicated by forward-looking statements. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include, among others, the risks and uncertainties inherent in Hansen Medical's business, including potential safety and regulatory issues that could slow or suspend its sales; its ability to effectively sell, service and support its products; the scope and validity of intellectual property rights applicable to its products; competition from other companies and its ability to obtain additional financing to support its operations. These and other risks are described in greater detail under the heading "Risk Factors" contained in Hansen Medical's periodic SEC filings, including its Quarterly Report on Form 10-Q filed with the SEC on May 8, 2009. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Hansen Medical undertakes no obligation to revise or update information herein to reflect events or circumstances in the future, even if new information becomes available.
Hansen Medical, Heart Design, Hansen Medical & Heart Design, Sensei and IntelliSense are registered trademarks, and Artisan, CoHesion, Fine Force Technology and Instinctive Motion are trademarks of Hansen Medical Inc., in the United States and other countries.
EnSite is a trademark of St. Jude Medical.
(1) Okumura Y, Johnson S, Packer D. An analysis of catheter tip/tissue contact force induced distortion of three-dimensional electroanatomical mapping created using the Sensei Robotic Catheter System. Heart Rhythm 2007;4:S318
(2) Kautzner J, Peichl P, Cihak R, Wichterle D, Mlcochova H. Early
Experience with Robotic
Navigation for Catheter Ablation of Paroxysmal Atrial Fibrillation. Pacing
and Clinical
Electrophysiology. 2009; 32: S1, p S163-S166.
Media Contact:
Amy Cook
925.552.7893
Email Contact
Investor Contact:
Steven Van Dick
650.404.5800
Email Contact