EyePoint Pharmaceuticals, Inc. announced updated interim data from the “Durasert® and Vorolanib in Ophthalmology” Phase 1 clinical trial of EYP-1901, a bioerodible sustained delivery intravitreal anti-vascular endothelial growth factor treatment targeting wet age-related macular degeneration.
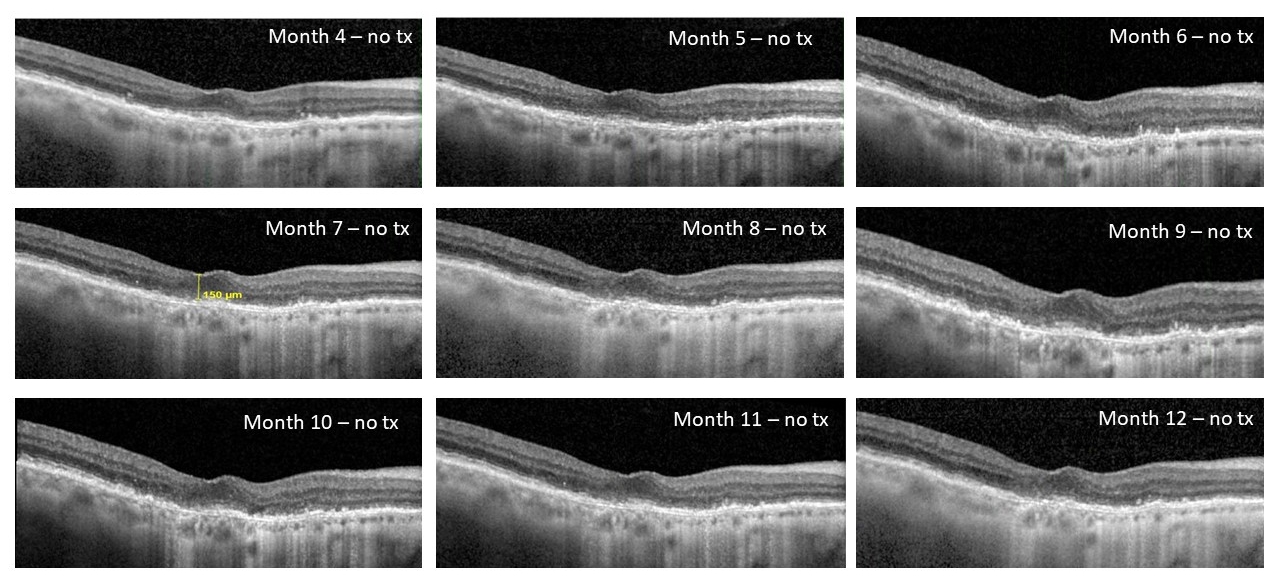
- Continued positive efficacy and durability with stable visual acuity (VA) and optical coherence tomography (OCT) through eight-month follow-up
- 41% of eyes remained rescue free up to nine months after a single dose of EYP-1901
- Positive safety data continued with no dose limiting toxicities, no ocular serious adverse events (SAEs) and no drug-related systemic SAEs observed
- Overall treatment burden reduced by 75% at eight months
- Phase 2 studies for EYP-1901 in wet AMD expected to initiate in Q3 2022 and in diabetic retinopathy in 2H 2022
WATERTOWN, Mass., Feb. 12, 2022 /PRNewswire/ -- EyePoint Pharmaceuticals, Inc. (NASDAQ: EYPT), a pharmaceutical company committed to developing and commercializing therapeutics to improve the lives of patients with serious eye disorders, today announced updated interim data from the "Durasert® and Vorolanib in Ophthalmology" (DAVIO) Phase 1 clinical trial of EYP-1901, a bioerodible sustained delivery intravitreal anti-vascular endothelial growth factor (anti-VEGF) treatment targeting wet age-related macular degeneration (wet AMD). These data are being presented today at the Angiogenesis, Exudation, and Degeneration 2022 virtual meeting by Jay S. Duker, M.D., Chief Operating Officer, EyePoint Pharmaceuticals.
Experience the interactive Multichannel News Release here: https://www.multivu.com/players/English/9013151-eyepoint-pharmaceuticals-phase-1-davio-clinical-study/
"Today's DAVIO clinical trial update further reinforces the potential for EYP-1901 as a durable anti-VEGF treatment in wet AMD," said Dr. Duker. "We are extremely pleased with the continued positive safety profile and strong durability results observed after a single injection of EYP-1901. We look forward to initiating a Phase 2 trial of EYP-1901 in the third quarter of this year to potentially alter the treatment paradigm for the millions of patients suffering from wet AMD."
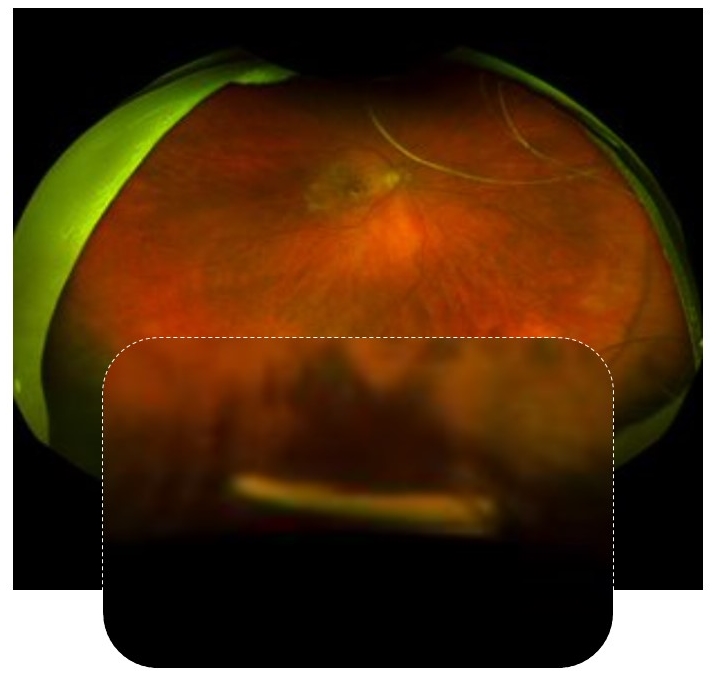
The interim eight-month follow-up data presented from the Phase 1 DAVIO clinical trial continue to show no reports of ocular serious adverse events (SAEs) or drug-related systemic SAEs. There have been no reported events of vitreous floaters, endophthalmitis, retinal detachment, implant migration in the anterior chamber, retinal vasculitis, or posterior segment inflammation. These data also show that 41% of eyes did not require any supplemental anti-VEGF injections up to nine months following a single dose of EYP-1901. Additionally, updated data from the eight-month follow-up confirm stable best corrected visual acuity (BCVA) (-3.0 ETDRS letters), stable central subfield thickness (CST) on optical coherence tomography (OCT) (+13 μm), and a clinically significant 75% reduction in treatment burden.
The Phase 1 DAVIO trial is an open-label, dose escalation clinical trial of EYP-1901 that enrolled 17 patients with previously treated wet AMD. EYP-1901 is a sustained delivery anti-VEGF investigational treatment that utilizes a bioerodible formulation of EyePoint's Durasert® drug delivery technology that has been utilized in four FDA-approved products, including EyePoint's YUTIQ® for chronic non-infectious uveitis affecting the posterior segment of the eye.
EyePoint plans to initiate a Phase 2 trial of EYP-1901 in wet AMD in the third quarter of this year, informed by a positive Type C meeting with U.S. Food and Drug Administration (FDA) in December 2021. The trial is expected to enroll 144 patients, randomly assigned to one of two doses of EYP-1901 or aflibercept control, with the primary efficacy endpoint of change in BCVA and secondary endpoints of change in CST, as measured by OCT, time to supplemental anti-VEGF injection and safety.
About EYP-1901
EYP-1901 is being developed as an investigational six-month treatment, initially in wet AMD, combining a bioerodible formulation of EyePoint's proprietary Durasert® sustained delivery technology with vorolanib, a tyrosine kinase inhibitor. Positive interim eight-month safety data from the ongoing Phase 1 DAVIO clinical trial of EYP-1901 show no reports of ocular or drug-related systemic SAEs and no dose limiting toxicities. Positive efficacy data from the ongoing DAVIO trial reflect positive efficacy and durability with stable VA and OCT through eight-month follow-up and 41% of eyes did not require any supplemental anti-VEGF injections up to nine months following a single dose of EYP-1901. Phase 2 studies are planned for wet AMD in Q3 2022 and in diabetic retinopathy in 2H 2022. Vorolanib, is licensed to EyePoint exclusively by Betta Pharmaceuticals for the potential treatment of multiple retinal diseases
About Wet AMD
Age-related macular degeneration (AMD) impacts as many as 11 million Americans. About 15% of those affected have neovascular or wet AMD - the hallmark of which is fluid and bleeding in the center of the retina, which may lead to irreversible vision loss. The majority of patients with wet AMD require intravitreal injections every month or two to control the disease. This intense treatment regimen represents an ongoing challenge for patients, caregivers, and physicians.
About EyePoint Pharmaceuticals, Inc.
EyePoint Pharmaceuticals (Nasdaq: EYPT) is a pharmaceutical company committed to developing and commercializing therapeutics to help improve the lives of patients with serious eye disorders. The Company's pipeline leverages its proprietary Durasert® technology for sustained intraocular drug delivery including EYP-1901, a potential six-month intravitreal anti-VEGF treatment initially targeting wet age-related macular degeneration. Durasert's proven intravitreal drug delivery platform has been safely administered to thousands of patients' eyes across four U.S. FDA approved products, including YUTIQ® for the treatment of chronic non-infectious uveitis affecting the posterior segment of the eye, which is currently marketed by the Company. EyePoint Pharmaceuticals is headquartered in Watertown, Massachusetts.
SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION ACT OF 1995: To the extent any statements made in this press release deal with information that is not historical, these are forward-looking statements under the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements regarding the potential for EYP-1901 as a six-month sustained delivery intravitreal anti-VEGF treatment targeting wet AMD, with potential in DR and RVO; our expectations regarding the timing and outcome of our Phase 1 DAVIO clinical trial for EYP-1901 for the potential treatment of wet AMD; our expectations regarding the timing and clinical development of our product candidates, including EYP-1901 and YUTIQ 50; and the potential advantages of our product candidates for the treatment of eye diseases; and other statements identified by words such as "will," "potential," "could," "can," "believe," "intends," "continue," "plans," "expects," "anticipates," "estimates," "may," other words of similar meaning or the use of future dates. Forward-looking statements by their nature address matters that are, to different degrees, uncertain. Uncertainties and risks may cause EyePoint's actual results to be materially different than those expressed in or implied by EyePoint's forward-looking statements. For EyePoint, this includes uncertainties regarding the timing and clinical development of our product candidates, including EYP-1901; the potential for EYP-1901 as a novel six-month treatment for serious eye diseases, including wet age-related macular degeneration, diabetic retinopathy and retinal vein occlusion; the effectiveness and timeliness of clinical trials, and the usefulness of the data; the timeliness of regulatory approvals; the continued impact of the COVID-19 pandemic on EyePoint's business, the medical community and the global economy and the impact of general business and economic conditions; the success of current and future license agreements; our dependence on contract research organizations, co-promotion partners, and other outside vendors and service providers; effects of competition and other developments affecting sales of our commercialized products, YUTIQ® and DEXYCU®; market acceptance of our products; product liability; industry consolidation; compliance with environmental laws; risks and costs of international business operations; volatility of stock price; possible dilution; absence of dividends; protection of our intellectual property and avoiding intellectual property infringement; retention of key personnel; manufacturing risks; and other factors described in our filings with the Securities and Exchange Commission. We cannot guarantee that the results and other expectations expressed, anticipated or implied in any forward-looking statement will be realized. A variety of factors, including these risks, could cause our actual results and other expectations to differ materially from the anticipated results or other expectations expressed, anticipated or implied in our forward-looking statements. Should known or unknown risks materialize, or should underlying assumptions prove inaccurate, actual results could differ materially from past results and those anticipated, estimated or projected in the forward-looking statements. You should bear this in mind as you consider any forward-looking statements. Our forward-looking statements speak only as of the dates on which they are made. We do not undertake any obligation to publicly update or revise our forward-looking statements even if experience or future changes makes it clear that any projected results expressed or implied in such statements will not be realized.
Investors:
Christina Tartaglia
Stern IR
Direct: 212-698-8700
christina.tartaglia@sternir.com
Media Contact:
Amy Phillips
Green Room Communications
Direct: 412-327-9499
Aphillips@greenroompr.com

SOURCE EyePoint Pharmaceuticals, Inc.
Company Codes: NASDAQ-NMS:EYPT




