San Francisco-based Audentes Therapeutics signed a licensing agreement and collaboration deal with Nationwide Children’s Hospital to expand its pipeline for vectorized antisense treatments for Duchenne muscular dystrophy (DMD) and myotonic dystrophy type 1 (DM1).
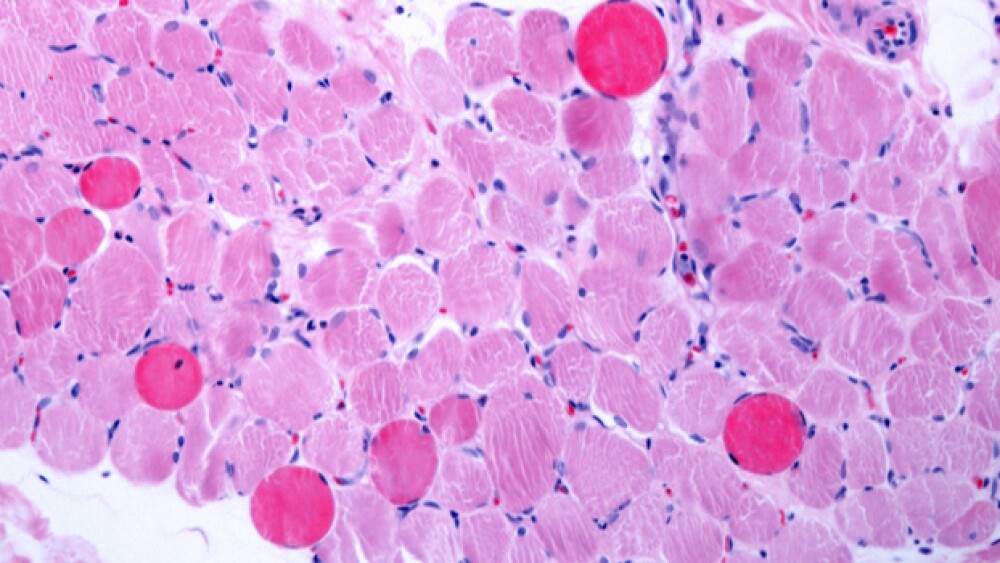
The key pathologic change of Duchenne muscular dystrophy is the myonecrosis. At an early phase, necrotic fibers appear swollen, homogeneous and deeply eosinophilic.
San Francisco-based Audentes Therapeutics signed a licensing agreement and collaboration deal with Nationwide Children’s Hospital to expand its pipeline for vectorized antisense treatments for Duchenne muscular dystrophy (DMD) and myotonic dystrophy type 1 (DM1).
Audentes and Nationwide Children’s are working together to develop AT702, an AAV-antisense therapy candidate engineered to induce exon 2 skipping for DMD with duplications of exon 2 and mutations in exons 1-5 of the dystrophin gene. DMD is a muscle-wasting disease that primarily affects boys and often leads to early death in the twenties. It is caused by various mutations in the dystrophin gene, which is involved in muscle development.
Vectorized exon skipping utilizes an adeno-associated virus (AAV) vector to deliver an antisense sequence that is designed to cause cells to skip over mutations or misaligned sections of the genetic code. This can lead to the expression of a more complete, functional protein, although the protein is often shortened or truncated.
As part of the deal, Audentes will also gain the expertise of two Nationwide Children’s researchers, Kevin M. Flanigan and Nicolas S. Wein.
“Today’s announcement represents a significant step forward in expanding our scientific platform and deepening our pipeline of product candidates for neuromuscular diseases with high unmet medical need,” stated Matthew R. Patterson, chairman and chief executive officer of Audentes. “We see tremendous potential in combining AAV with validated oligonucleotide-based approaches to treat diseases that are not amenable to traditional AAV-based gene replacement. We believe this approach, combined with our in-house large-scale cGMP manufacturing capability, can deliver best-in-class therapies for the treatment of Duchenne muscular dystrophy and myotonic dystrophy.”
In addition to developing AT702, separate from the Nationwide Children’s collaboration, Audentes is running preclinical studies to advance AT751 and AT753, also vectorized exon skipping candidates, to treat DMD patients with genotypes involving exon 51 and exon 53 skipping. Both use the same vector construct backbone as AT702, which may speed clinical development.
With these three programs, Audentes is targeting more than 25% of DMD patients. It hopes that by leveraging its vectorized exon-skipping platform, it can develop enough products to treat up to 80% of DMD patients.
Audentes and Nationwide Children’s are also evaluating vectorized RNA knockdown and vectorized exon skipping for DM1. They are designed to stop the accumulation of toxic dystrophia myotonica-protein kinase (DMPK) RNA in cells, restoring normal cellular function. Preclinical studies are ongoing and Audentes is planning an Investigational New Drug (NDA) application for AT466 in 2020.
In October 208, Sarepta Therapeutics, based in Cambridge, Mass., signed a discovery and development deal with Nationwide Children’s Hospital. This deal gave Sarepta the exclusive option to a Nationwide Children’s gene therapy candidate, neurotrophin 3 (NT-3), to treat Charcot-Marie-Tooth (CMT) neuropathies, including CMT type 1A. CMT is a family of hereditary, degenerative nerve diseases that affect motor skills. It is the most common inherited neuromuscular disorder, affecting over 2.8 million people around the world.
In 2016, the U.S. Food and Drug Administration (FDA) approved the first drug to treat DMD, Sarepta’s Exondys 51. It was a long, dramatic and controversial approval journey involving numerous public hearings, internal FDA battles and letters from Congress and leading DMD physicians to the agency.
These deals mark the entry of gene therapy into mainstream drug development. Roche recently acquired Spark Therapeutics for $4.8 billion. Spark developed a gene therapy for a rare eye disease and hemophilia. And in 2018, Novartis acquired AveXis for $8.7 billion. AveXis has a gene therapy for spinal muscular atrophy (SMA).
Marshall Gorden, a senior research analyst at ClearBridge Investments, told Barron’s last year, “Big drug companies are waking up and saying this is a real technology and that they need to be there.”