Sideromics Completes Pre-IND Meeting With FDA On Siderocillin For Two Indications: MRSA Bacteremia And ABSSSI
IRVINE, Calif., April 8, 2014 /PRNewswire/ -- Sideromics LLC, a pharmaceutical company specializing in the development of novel antimicrobial compounds, announced today that the company has successfully completed a Type B pre-investigational new drug (PIND) meeting with the U.S. Food and Drug Administration (FDA) Division of Anti-Infective Products (DAIP). The purpose of the meeting was to obtain FDA concurrence on the nonclinical, clinical, and regulatory pathway for the development of Siderocillin, a novel first-in-class antimicrobial agent.
Sideromics' Siderocillin, is a first-in-class antibiotic within the Sideromycin group. Siderocillin employs a completely new and novel antimicrobial approach. Sideromics is pursuing multiple indications under this IND program:
- Treatment of patients with hospital-acquired bacteremia due to methicillin-resistant Staphylococcus aureus (MRSA)
- Treatment of hospitalized patients with acute bacterial skin and skin structure tissue infections (ABSSSI)
Sideromics is currently preparing a GMP batch of Siderocillin, which will undergo a microbiological testing program discussed with the FDA. The PIND meeting with the FDA confirmed that the initial IND for Siderocillin will not require additional nonclinical safety data to support our first phase I single dose trial in humans. This enables Sideromics to enter the clinic promptly.
As part of the PIND meeting program, the FDA asked Sideromics to consider requesting designation of Siderocillin as a "Qualified Infectious Disease Product" (QIDP), as well as requesting fast track designation at the time of QIDP designation.
QIDP designation is part of the Generating Antibiotic Incentives Now (GAIN) statute, created by Congress to encourage the development of therapies for drug-resistant organisms known to cause serious or life-threatening infections. The GAIN provisions are included in the FDA Safety and Innovation Act (FDASIA) that was signed into law in July 2012. The GAIN act is a legislative effort to incentivize the development of new antibiotic agents that target serious life-threatening infections. Siderocillin is clearly the type of novel compound needed in medicine's current losing battle with multiresistant organisms.
By expediting and fast tracking the FDA review process, the QIDP designation will allow Sideromics to bring Siderocillin to patients with serious infections much more quickly. QIDP status will also complement Sideromics' patent portfolio with an additional total of 10 years exclusivity in the U.S.
Research and development of antibiotics has declined in recent years and the pipeline for antibiotics today is significantly smaller than in the past. In 1990, there were 18 major pharmaceutical companies engaged in antibiotics research, today there are only four. Given the speed at which microbes have become resistant in recent years, the innovation in antibiotics research has not been successful in addressing this growing problem.
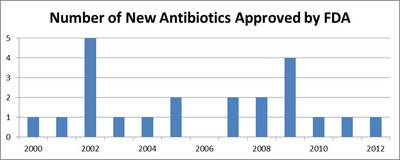
"This is a serious problem for physicians and health care workers," says Sharon Moalem MD, PhD, Sideromics cofounder and director of research, "It's like trying to fight a fire with one hand tied behind your back." As the number of new antibiotics approved by the FDA has been declining over the last few decades, the number of individuals in the U.S. dying from infections caused by multiresistant microbes, such as MRSA has been on a steep rise.
In the latest reports (March 2014) from Centers for Disease Control and Prevention (CDC), the agency found that on any given day, approximately one in 25 patients has at least one infection contracted during the course of their hospital care. In 2011 alone, 722,000 infections were recorded according to new data from CDC.
"Our companies mandate was to aggressively screen compounds that target bacteria that are the so called 'superbugs' or multiresistant microbes. We discovered Siderocillin by studying how rare human conditions evolved to protect us from microbial infections," says Moalem.
Using their first-in-class intellectual property compounds, with clinical trials set to begin by the end of 2014, Sideromics is poised to make dramatic changes to the antimicrobial landscape.
About Sideromics
Sideromics is a pharmaceutical company developing novel compounds and methods to treat or prevent diseases and disorders caused by pathogenic microorganisms, such as bacteria, fungi, and parasites. Sideromics has developed a pipeline of products with possible indications in humans and domestic animals, as well as for use in food-animal growth & processing. Sideromics' pipeline of products range from early proof of concept through advance stages of development. Current and pending patents and IP cover a broad range of applications in the U.S. and on a global basis.
Photo - http://photos.prnewswire.com/prnh/20140408/LA99615
CONTACT: | Sideromics LLC |
Investor & Public Relations: | |
Stacey Lense Email: stacey.lense@sideromics.com | |
Rachel Stone Email: rachel.stone@sideromics.com | |
(800) 552-1680 |
SOURCE Sideromics LLC
Help employers find you! Check out all the jobs and post your resume.
