Groundbreaking XT MicroSlides Deliver Accurate Results from 2 Simultaneous Miniaturized Tests, Simplifying Workflow and Improving Turnaround Time in the Lab
Groundbreaking XT MicroSlides Deliver Accurate Results from 2 Simultaneous Miniaturized Tests, Simplifying Workflow and Improving Turnaround Time in the Lab
RARITAN, N.J., April 9, 2019 /PRNewswire/ -- Ortho Clinical Diagnostics' VITROS® XT MicroSlides, featuring new, multi-test technology that allows labs to run two tests simultaneously on one MicroSlide, have been cleared for market by the U.S. Food and Drug Administration. The product pairs tests that physicians typically order together, while maintaining the high-quality results that physicians expect from Ortho's MicroSlide technology. Available test pairs include: triglycerides and cholesterol; urea and creatinine; and glucose and calcium.
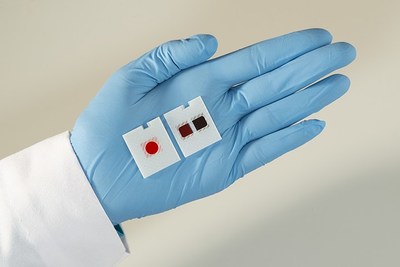
By pairing commonly ordered tests so they can be run simultaneously, Ortho's VITROS® XT MicroSlides, which are available for use on Ortho's VITROS® XT 7600 Integrated System, improve performance and productivity in the clinical lab, enabling a higher test throughput without requiring additional or larger analyzers. This is a significant benefit in a hospital environment, where space is often at a premium. In addition, the miniaturized testing areas on each MicroSlide require less patient blood sample for each of the paired tests, an important advantage for vulnerable patients or those with venous access issues. Ortho's VITROS® XT MicroSlides are powered by DIGITAL CHEMISTRY™ technology, available in the VITROS® XT 7600 Integrated System, which uses advanced optics to glean significantly more information from each test than was possible before.
"Labs today are challenged to run increasing volumes of tests in shorter timeframes, with less physical space available for larger analyzers," said Michael Iskra, head of Ortho's North America operations. "Because they enable the simultaneous running of two tests that are typically ordered together, our VITROS XT MicroSlides address these numerous challenges with one elegant solution."
Ortho's VITROS® XT MicroSlides, which include VITROS® XT UREA-CREA, TRIG-CHOL and GLU-Ca Slides, have been cleared by the U.S. Food and Drug Administration. In addition, they have received CE Mark, signifying conformance to applicable European Union regulatory requirements. These products are available on the VITROS® XT 7600 Integrated System in numerous countries, including the U.S., Canada, Chile, France, Germany, Hong Kong, Iceland, Italy, Spain, Switzerland, the U.K., Australia, Liechtenstein, Norway and New Zealand.
For more information, follow Ortho on LinkedIn, Twitter, Facebook and Instagram.
About Ortho's VITROS® Systems
The Ortho VITROS® Chemistry, Immunodiagnostics and Integrated Systems from Ortho Clinical Diagnostics is a portfolio of products and patented enabling technologies which help clinical laboratories diagnose, monitor and treat disease. Ortho's VITROS® Products are engineered to help clinical laboratories with organizational, operational and economic challenges.
About Ortho Clinical Diagnostics
Ortho Clinical Diagnostics is a global leader of in vitro diagnostics serving the clinical laboratory and immunohematology communities. Across hospitals, hospital networks, blood banks and labs in more than 125 countries and territories, Ortho's high-quality products and services enable health care professionals to make better-informed treatment decisions. For the immunohematology community, Ortho's blood typing products help ensure every patient receives blood that is safe, the right type and the right unit. Ortho brings sophisticated testing technologies, automation, information management and interpretation tools to clinical laboratories around the world to help them run more efficiently and effectively and improve patient care. Ortho's purpose is to improve and save lives with diagnostics, and it does that by reimagining what's possible. This is what has defined Ortho for more than 75 years, and it's what drives Ortho forward. For more information, visit www.orthoclinicaldiagnostics.com.
© Ortho Clinical Diagnostics 2019
PR-04942

![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/fda-clears-vitros-xt-chemistry-product-slides-from-ortho-clinical-diagnostics-300826485.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/fda-clears-vitros-xt-chemistry-product-slides-from-ortho-clinical-diagnostics-300826485.html
SOURCE Ortho Clinical Diagnostics




