United States
After suffering in the wake of expired tax incentives for pharmas, the island is trying to take advantage of geopolitics to grow its drug manufacturing sector.
Looking for an IT job? From data engineer to information security, check out the BioSpace list of 10 companies hiring life sciences professionals like you.
Looking for a research and development job? Check out the BioSpace list of 12 companies hiring life sciences professionals like you.
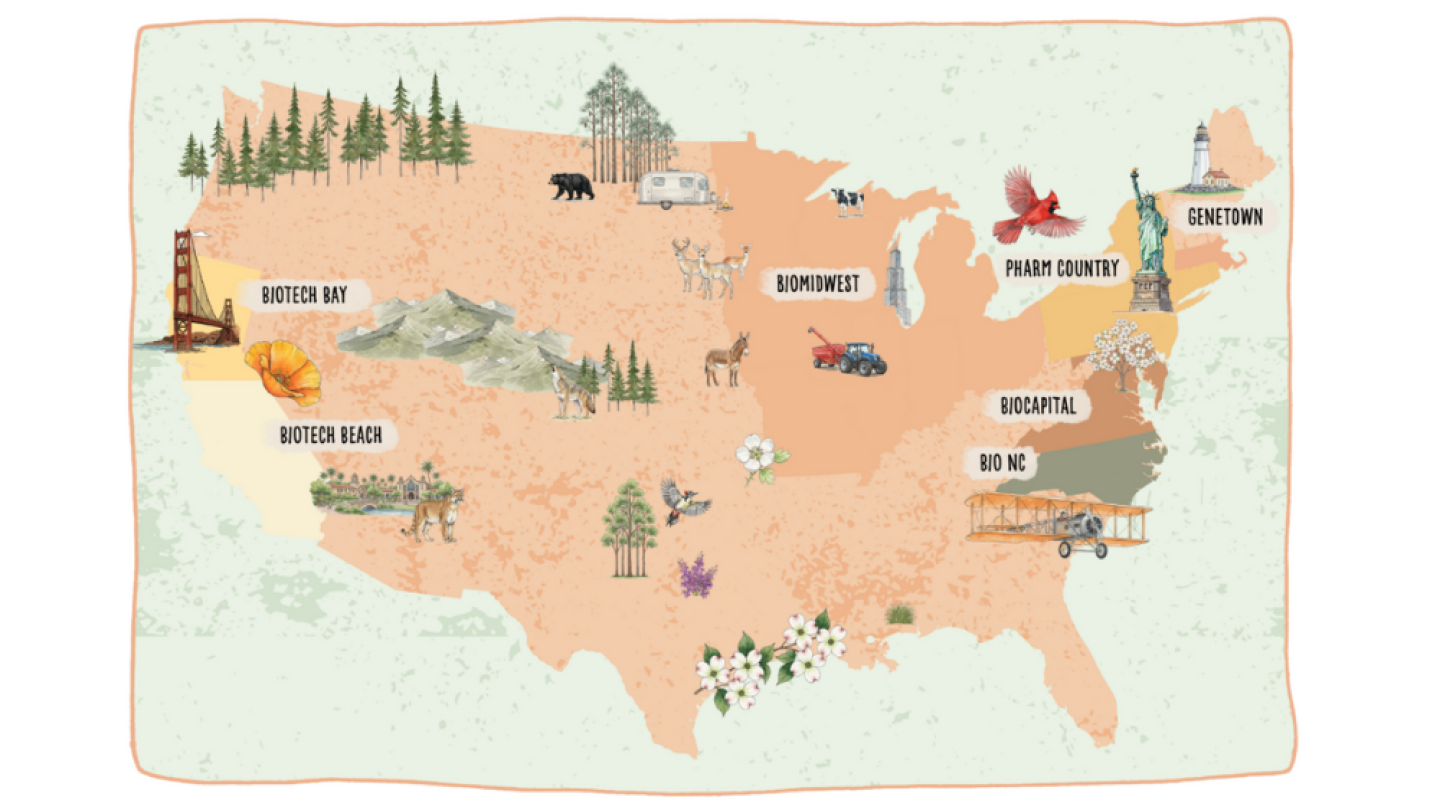
BioSpace has revealed its new 2026 Hotbed Maps, showcasing seven regional life sciences hot spots.
Less than six months after cutting 20% of its employees, Vedanta Biosciences has again laid off staff. According to one affected staffer, half of the Cambridge, Massachusetts–based biotech’s workforce is being cut while most of the rest are furloughed.
Looking for a manufacturing job? Check out the BioSpace list of 11 companies hiring life sciences professionals like you.
The past year saw manufacturing challenges pushed into the spotlight. BioSpace spoke to two executives who shared key issues facing those working in this area, from finding the right providers to dealing with regulatory uncertainty.
Acadia Pharma’s Catherine Owen Adams is one of the founders of a group of small- to mid-cap biotechs advocating against a ‘peanut butter blanket’ approach to drug pricing for small companies.
In 2025, made or projected biopharma workforce cuts affected about 42,700 employees, according to BioSpace tallies. BioSpace takes a deep dive into which companies and locations were impacted and speaks to experts about what to expect ahead—and why.
If you’re looking for a biopharma job in Philadelphia, here are eight companies on BioSpace hiring right now.
PRESS RELEASES