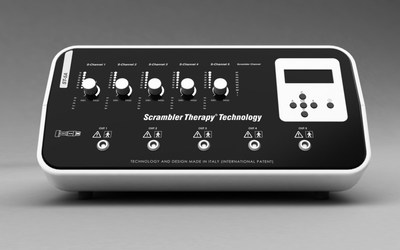
The next generation Scrambler Therapy® Technology ST-5A has received marketing approval from the FDA.
ROME, Feb. 17, 2021 /PRNewswire/ -- The next generation Scrambler Therapy® Technology ST-5A has received marketing approval from the FDA (K201458). This new device replaces the previous two generations known as "Calmare®" and "Scrambler Therapy® Technology MC-5A" respectively, which are no longer in production.
Indications for Use (MDR 745/2017)
The specific indications for use are clinical results from scientific literature indexed and published in peer review scientific journals, based exclusively on this technology are:
- Symptomatic relief of chronic opioid-resistant pain.
- Symptomatic relief of chronic neuropathic and cancer pain.
In general, this device is indicated for all forms of severe chronic pain, specifically pain that does not respond to other pharmacological or non-pharmacological treatments, including opioids.
This third generation medical device is made entirely in Italy, where the scientific research that generated this technology originated. Among the most significant innovations are a modernized design, integrated compatibility dual power supply, quality guaranteed by the strict European Medical Device Regulation, and the stylistic and technological tradition of "Made in Italy".
About Delta International Service & Logistics
DIS&L is the agency created for the international development of Scrambler Therapy® Technology, and the selection of Scrambler Therapy Technology ST-5A Medical Device distributors in all international areas. DIS&L through its law firm is the only company authorized to sign international exclusive agreements, provide maintenance and client support for technical treatment training and other logistical needs.
About Scrambler Therapy®
Scrambler Therapy® scientific research and development technology has been developed in Italy by Professor Giuseppe Marineo, who is the sole owner of its intellectual property rights. The official "Scrambler Therapy"® scientific and clinical information website is:
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/us--grants-marketing-approval-for-the-new-scrambler-therapy-technology-st-5a-medical-device-for-the-non-invasive-treatment-of-neuropathic-and-cancer-pain-mdr-7452017-301229199.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/us--grants-marketing-approval-for-the-new-scrambler-therapy-technology-st-5a-medical-device-for-the-non-invasive-treatment-of-neuropathic-and-cancer-pain-mdr-7452017-301229199.html
SOURCE Delta International Services & Logistics s.r.l.