Year two results consistent with previously announced key secondary endpoint data on retinal fluid (IRF and/or SRF) showing superior reductions versus aflibercept
- Year two results consistent with previously announced key secondary endpoint data on retinal fluid (IRF and/or SRF) showing superior reductions versus aflibercept
- Superior reductions in central subfield thickness demonstrated at year one were reaffirmed at year two with brolucizumab 6 mg versus aflibercept
- Robust visual gains shown in year one with brolucizumab were maintained in year two
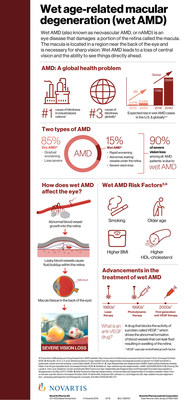
EAST HANOVER, N.J., Oct. 27, 2018 /PRNewswire/ -- Novartis announced additional brolucizumab Phase III results from year two that reaffirmed its positive year one findings. Brolucizumab met its primary endpoint of non-inferiority versus aflibercept in best corrected visual acuity (BCVA) and exhibited superiority in key retinal outcomes at year one (48 weeks)1,2. Secondary endpoints at year two (96 weeks) reaffirmed superiority of brolucizumab 6 mg in reduction of retinal fluid, an important marker of disease activity in patients with neovascular age-related macular degeneration (nAMD)1,3. Approximately 20 to 25 million people are affected by nAMD, also known as wet AMD, a leading cause of blindness worldwide4,5.
The year two HAWK and HARRIER findings demonstrated that fewer patients with nAMD had intra-retinal fluid (IRF) and/or sub-retinal fluid (SRF) — key markers used by physicians to determine injection frequency in clinical practice — with brolucizumab 6 mg versus aflibercept at week 96 [24% for brolucizumab 6 mg vs. 37% for aflibercept in HAWK (P=0.0001); 24% vs. 39%, respectively, in HARRIER (P<0.0001)]1*.
Additionally, brolucizumab 6 mg patients continued to demonstrate reductions in central subfield thickness (CST) at week 961. An increase in CST in nAMD is an important measure of abnormal fluid accumulation and edema and may result in reduced vision. Absolute reductions in CST from baseline were ‑175 µm for brolucizumab 6 mg versus ‑149 µm for aflibercept in HAWK (P=0.0057) and ‑198 µm versus ‑155 µm, respectively, in HARRIER (P<0.0001)1*.
Also at week 96, fewer brolucizumab 6 mg patients had sub-retinal pigment epithelium (sub-RPE) fluid (11% for brolucizumab 6 mg vs. 15% for aflibercept in HAWK; 17% vs. 22%, respectively, in HARRIER)1. Additionally, of patients on brolucizumab 6 mg who successfully completed year one on a 12-week dosing interval, 82% in HAWK and 75% in HARRIER were maintained on a 12-week dosing interval in year two1.
"These findings at year two reaffirm the excellent year one brolucizumab data regarding retinal fluid reduction, a key goal for physicians treating patients with nAMD," said Dr. Pravin U. Dugel, Managing Partner, Retinal Consultants of Arizona; Clinical Professor, Roski Eye Institute, Keck School of Medicine, University of Southern California; and principal investigator of both trials. "These consistent results continue to support brolucizumab as a potential new treatment for patients with nAMD."
As previously announced, HAWK and HARRIER met their primary endpoint of non-inferiority in mean change in BCVA at week 48 with brolucizumab versus aflibercept2. Brolucizumab maintained robust visual gains in year two, with mean change in BCVA of 5.9 letters for brolucizumab 6 mg versus 5.3 letters for aflibercept in HAWK, and 6.1 letters versus 6.6 letters, respectively, in HARRIER1.
"Over two years, brolucizumab consistently dried retinal fluid better than aflibercept; additionally, the robust visual gains shown in year one with brolucizumab were maintained in year two," said Shreeram Aradhye, Global Head Medical Affairs and Chief Medical Officer, Novartis Pharmaceuticals. "With sustained improvements in key anatomical outcomes that denote disease activity, brolucizumab is an important scientific advance and underscores our commitment to reimagining medicine."
No new, previously unreported types of safety events were identified at week 96, and brolucizumab continued to be comparable to aflibercept with the overall incidence of adverse events balanced across all treatment groups in both studies1. The most frequent ocular adverse events (≥5% of patients in any treatment arm) were reduced visual acuity, conjunctival hemorrhage, vitreous floaters, eye pain, dry eye, retinal hemorrhage, cataract and vitreous detachment1. The most frequent non-ocular adverse events were typical of those reported in a nAMD population; there were no notable differences between arms1.
These new 96-week data, based on pre-specified secondary endpoints from the HAWK and HARRIER trials, were presented at the American Academy of Ophthalmology (AAO) 2018 Annual Meeting as a follow-up to the year one data presented in November 20171,2.
About brolucizumab (RTH258)
Brolucizumab (RTH258) is a humanized single-chain antibody fragment (scFv) and the most clinically advanced, humanized single-chain antibody fragment to reach this stage of development. Single-chain antibody fragments are highly sought after in drug development due to their small size, enhanced tissue penetration, rapid clearance from systemic circulation and drug delivery characteristics6,7,8.
The proprietary innovative structure results in a small molecule (26 kDa) with potent inhibition of, and high affinity to, all VEGF-A isoforms6,9. In preclinical studies, brolucizumab inhibited activation of VEGF receptors through prevention of the ligand-receptor interaction6,8,9. Increased signaling through the VEGF pathway is associated with pathologic ocular angiogenesis and retinal edema10. Inhibition of the VEGF pathway has been shown to inhibit the growth of neovascular lesions, resolve retinal edema and improve vision in patients with chorioretinal vascular diseases11.
About HAWK and HARRIER study design
With more than 1,800 patients across 400 centers worldwide, HAWK (NCT02307682) and HARRIER (NCT02434328) are the first and only global head-to-head trials in patients with nAMD that prospectively demonstrated efficacy at week 48 using an innovative q12w/q8w regimen, with a majority of patients on q12w immediately following the loading phase2,12,13. Both studies are 96-week prospective, randomized, double-masked multi-center studies and part of the Phase III clinical development of brolucizumab12,13.
The studies were designed to compare the efficacy and safety of intravitreal injections of brolucizumab 6 mg (HAWK and HARRIER) and 3 mg (HAWK only) versus aflibercept 2 mg in patients with nAMD12,13. In both trials, patients were randomized to either brolucizumab or aflibercept. Immediately following the 3-month loading phase, patients in the brolucizumab arms received a q12w dosing interval with an option to adjust to a q8w dosing interval based on masked disease activity assessments at defined visits. Aflibercept was dosed bi-monthly according to its label at the time of study initiation2,12,13.
Brolucizumab met the primary efficacy objective of non-inferiority versus aflibercept in mean change in best-corrected visual acuity (BCVA) from baseline to week 48 with high statistical significance2. Additionally, brolucizumab demonstrated superiority in three secondary endpoints considered key parameters of nAMD: central subfield retinal thickness, retinal fluid (intraretinal fluid and/or subretinal fluid) and disease activity2.
At year two, the most frequent ocular adverse events (≥5% of patients in any treatment arm) for brolucizumab 3 mg, 6 mg and aflibercept, respectively, in HAWK were conjunctival hemorrhage (10.9%, 8.1% and 8.9%), reduced visual acuity (9.5%, 6.1% and 8.1%), vitreous floaters (7.3%, 6.1% and 4.4%), eye pain (7.8%, 5.0% and 5.8%), retinal hemorrhage (3.9%, 5.8% and 5.6%), cataract (5.0%, 5.6% and 3.6%), vitreous detachment (6.7%, 5.3% and 5.3%) and dry eye (5.6%, 5.3% and 7.2%)1. The incidences of these events for brolucizumab 6 mg and aflibercept, respectively, in HARRIER were conjunctival hemorrhage (4.6% and 5.1%), reduced visual acuity (8.6% and 7.0%), vitreous floaters (4.1% and 1.4%), eye pain (3.5% and 5.1%), retinal hemorrhage (3.2% and 1.1%), cataract (3.0% and 11.7%), vitreous detachment (2.7% and 2.2%) and dry eye (2.7% and 3.0%)1.
About neovascular age-related macular degeneration (nAMD or wet AMD)
nAMD is the leading cause of severe vision loss and legal blindness in people over the age of 65 in North America, Europe, Australia and Asia, impacting an estimated 20 to 25 million people worldwide4,5. nAMD occurs when abnormal blood vessels form underneath the macula, the area of the retina responsible for sharp, central vision. These blood vessels are fragile and leak fluid, disrupting the normal retinal architecture and ultimately causing damage to the macula14,15,16.
Early symptoms of nAMD include distorted vision or metamorphopsia and difficulties seeing objects clearly17. Prompt diagnosis and intervention are essential. As the disease progresses, cell damage increases, further reducing vision quality. This progression can lead to a complete loss of central vision, leaving the patient unable to read, drive or recognize familiar faces14. Without treatment, vision can rapidly deteriorate18.
About Novartis in ophthalmology
For more than 70 years, patients, caregivers and healthcare providers worldwide have looked to Novartis for state-of-the-art treatments in eye diseases. We continue to invest in science as well as in strategic alliances to help ensure patients have access to screening, diagnosis, and our eye medicines. Our commitment to vision extends globally across ages, from premature infants to seniors, from rare diseases to those affecting millions, from eye drops to gene therapies. Our aspiration: reimagining eye care to help everyone see possibilities.
Disclaimer
This press release contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements can generally be identified by words such as "potential," "can," "will," "plan," "expect," "anticipate," "look forward," "believe," "committed," "investigational," "pipeline," "launch," or similar terms, or by express or implied discussions regarding potential marketing approvals, new indications or labeling for the investigational or approved products described in this press release, or regarding potential future revenues from such products. You should not place undue reliance on these statements. Such forward-looking statements are based on our current beliefs and expectations regarding future events, and are subject to significant known and unknown risks and uncertainties. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those set forth in the forward-looking statements. There can be no guarantee that the investigational or approved products described in this press release will be submitted or approved for sale or for any additional indications or labeling in any market, or at any particular time. Nor can there be any guarantee that such products will be commercially successful in the future. In particular, our expectations regarding such products could be affected by, among other things, the uncertainties inherent in research and development, including clinical trial results and additional analysis of existing clinical data; regulatory actions or delays or government regulation generally; global trends toward health care cost containment, including government, payor and general public pricing and reimbursement pressures; our ability to obtain or maintain proprietary intellectual property protection; the particular prescribing preferences of physicians and patients; general political and economic conditions; safety, quality or manufacturing issues; potential or actual data security and data privacy breaches, or disruptions of our information technology systems, and other risks and factors referred to in Novartis AG's current Form 20-F on file with the US Securities and Exchange Commission. Novartis is providing the information in this press release as of this date and does not undertake any obligation to update any forward-looking statements contained in this press release as a result of new information, future events or otherwise.
About Novartis
Novartis is reimagining medicine to improve and extend people's lives. As a leading global medicines company, we use innovative science and digital technologies to create transformative treatments in areas of great medical need. In our quest to find new medicines, we consistently rank among the world's top companies investing in research and development. Novartis products reach nearly 1 billion people globally and we are finding innovative ways to expand access to our latest treatments. About 125 000 people of more than 140 nationalities work at Novartis around the world. Novartis Pharmaceuticals Corporation, a US affiliate of Novartis, is located in East Hanover, NJ.
Novartis is on Twitter. Sign up to follow @Novartis at http://twitter.com/novartis
For Novartis multimedia content, please visit www.novartis.com/news/media-library
For questions about the site or required registration, please contact media.relations@novartis.com
References
1.
Dugel P, et al. Phase 3, randomized, double-masked, multi-center trials of brolucizumab versus aflibercept for neovascular AMD: 96-week results from the HAWK and HARRIER studies. Presented at: The American Academy of Ophthalmology on October 27, 2018, Chicago.
2.
Dugel P, et al. HAWK & HARRIER: 48-week results of 2 multi-centered, randomized, double-masked trials of brolucizumab versus aflibercept for neovascular AMD. Presented at: The American Academy of Ophthalmology on November 10, 2017, New Orleans.
3.
Arnold J et al. The role of sub-retinal fluid in determining treatment outcomes in patients with neovascular age-related macular degeneration--a phase IV randomised clinical trial with ranibizumab: the FLUID study. BMC Ophthalmol. 2016;143(4):679-680.
4.
Chopdar A, et al. Age related macular degeneration. BMJ. 2003;26(7387):485-488.
5.
Schmidt-Erfurth U, et al. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br J Ophthalmol. 2014;98:1144-1167.
6.
Escher D, et al. Single-chain antibody fragments in ophthalmology. Oral presentation at EURETINA congress. 2015. Abstract.
7.
Nimz EL, et al. Intraocular and systemic pharmacokinetics of brolucizumab (RTH258) in nonhuman primates. The Association for Research in Vision and Ophthalmology (ARVO) annual meeting. 2016. Abstract 4996.
8.
Gaudreault J, et al. Preclinical pharmacology and safety of ESBA1008, a single-chain antibody fragment, investigated as potential treatment for age related macular degeneration. ARVO Annual Meeting abstract. Invest Ophthalmol Vis Sci 2012;53:3025. http://iovs.arvojournals.org/article.aspx?articleid=2354604 (link is external). Accessed October 2018.
9.
Tietz J, et al. Affinity and Potency of RTH258 (ESBA1008), a Novel Inhibitor of Vascular Endothelial Growth Factor A for the Treatment of Retinal Disorders. IOVS. 2015; 56(7):1501.
10.
Qazi Y, et al. Mediators of ocular angiogenesis. J. Genet. 2009;88(4):495-515.
11.
Kim R. Introduction, mechanism of action and rationale for anti-vascular endothelial growth factor drugs in age-related macular degeneration. Indian J Ophthalmol. 2007;55(6):413-415.
12.
ClinicalTrials.gov. Identifier NCT02307682. Available at https://clinicaltrials.gov/ct2/show/NCT02307682 (link is external). Accessed October 2018.
13.
ClinicalTrials.gov. Identifier NCT02434328. Available at https://clinicaltrials.gov/ct2/show/NCT02434328 (link is external). Accessed October 2018.
14.
World Health Organization. Priority eye diseases: Age-related macular degeneration. Available at http://www.who.int/blindness/causes/priority/en/index7.html (link is external). Accessed October 2018.
15.
NHS Choices. Macular Degeneration. Available at http://www.nhs.uk/Conditions/Macular-degeneration/Pages/Introduction.aspx (link is external). Accessed October 2018.
16.
National Eye Institute. Facts About Age-Related Macular Degeneration. Available at https://nei.nih.gov/health/maculardegen/armd_facts (link is external). Accessed October 2018.
17.
NHS Choices. Macular degeneration - Symptoms. Available at http://www.nhs.uk/Conditions/Macular-degeneration/Pages/Symptoms.aspx (link is external). Accessed October 2018.
18.
van Lookeren Campagne M, et al. Mechanisms of age-related macular degeneration and therapeutic opportunities. J Pathol. 2014; 232(2):151-64. doi: 10.1002/path.4266.
Novartis Media Relations
Central media line: +41 61 324 2200
E-mail: media.relations@novartis.com
Eric Althoff
Novartis Global Media Relations
+41 61 324 7999 (direct)
+41 79 593 4202 (mobile)
Martin DeBenedetto
Director, Communications
(862) 778-7619 (direct)
(973) 738-4104 (mobile)
martin.debenedetto@novartis.com
Novartis Investor Relations
Central investor relations line: +41 61 324 7944
E-mail: investor.relations@novartis.com
North America
Richard Pulik
Cory Twining
+1 212 830 2448
+1 212 830 2417
*Descriptive P-values related to pre-specified secondary endpoints assessed at weeks 16 and 48
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/two-year-data-for-novartis-brolucizumab-reaffirm-superiority-versus-aflibercept-in-reducing-retinal-fluid-in-patients-with-namd-300739015.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/two-year-data-for-novartis-brolucizumab-reaffirm-superiority-versus-aflibercept-in-reducing-retinal-fluid-in-patients-with-namd-300739015.html
SOURCE Novartis




