ROCKVILLE, Md., Dec. 8, 2015 /PRNewswire/ -- Synthetic Biologics, Inc. (NYSE MKT: SYN), a clinical stage company focused on developing therapeutics to protect the gut microbiome, announced positive topline results from the four week Phase 2 clinical trial of SYN-010 for the treatment of irritable bowel syndrome with constipation (IBS-C).
"We are pleased to report that SYN-010, a proprietary modified-release formulation of lovastatin lactone, lowered breath methane and improved stool frequency in patients with IBS-C," stated Joseph Sliman, M.D., MPH, Senior Vice President, Clinical & Regulatory Affairs of Synthetic Biologics.
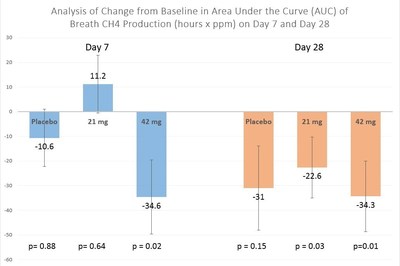
"Methane is believed to be a causative factor in constipation, and the primary objective of this trial was to ascertain whether SYN-010 could lower breath methane either at the 7 or 28 day level. The results show that high dose SYN-010 (42 mg) lowered methane at the 7-day and 28-day time point, and low dose SYN-010 (21 mg) lowered methane after 28 days. The inserted graph, which is modified from the scientific abstract we submitted to Digestive Disease Week 2016, depicts methane decreases within dose groups before and after 7 or 28 days of treatment."
Dr. Sliman continued, "We were pleasantly surprised that, in addition to methane reduction, we saw improvements in clinical outcomes despite the small size of this study. We saw improvements in stool frequency response for the 21 mg dose group and in weekly abdominal pain intensity for the 42 mg dose group, both outcome measures are patterned after the FDA IBS guidance."
"The completion of this four week exploratory Phase 2 clinical trial for SYN-010 is an important milestone for Synthetic Biologics. Positive clinical outcomes in this study are encouraging and bring us closer to offering relief to the millions of people who suffer from the symptoms of IBS-C, while at the same time preserving the important balance of their microbiome," stated Jeffrey Riley, Chief Executive Officer.
Mr. Riley concluded, "The second Phase 2 clinical trial for SYN-010 is currently underway and is evaluating the higher dose of SYN-010 (42 mg) during an additional eight week period in patients who rolled over from the first study. We expect to report topline results from the second Phase 2 trial during the first quarter of 2016 and planning of the Phase 3 trial is underway. We are hosting a Microbiome Clinical Program Seminar in NYC on Thursday, December 10, 2015 where we will present the detailed results of this first Phase 2 trial, along with others relevant to our clinical development programs."
About the First SYN-010 Phase 2 Clinical Trial
SYN-010 is Synthetic Biologics' proprietary, modified-release formulation of lovastatin lactone that is intended to reduce methane production by certain microorganisms (M. smithii) in the gut while minimizing disruption to the microbiome. SYN-010 is intended to act primarily in the intestinal lumen, ideally limiting systemic absorption, thereby targeting a major cause of IBS-C, not just the symptoms.
In this four week Phase 2 study, 63 patients with IBS-C and a breath methane value > 10 parts per million at screening were randomly assigned to receive placebo, a 21 mg SYN-010 dose or a 42 mg SYN-010 dose orally once daily for 28 days. The primary endpoint was the change from baseline in the area under the curve (AUC) of breath methane production at day 7, based on a 180-minute lactulose breath test (LBT). Secondary efficacy assessments included change in methane AUC at day 28, stool frequency and consistency, abdominal pain, and safety data. A necessary normalization of the severely left-skewed breath test data, not prespecified in the statistical analysis plan, was accomplished by square root transformation; paired t-tests were performed allowing each subject to serve as their own control. The analyses of the clinical outcomes were performed with untransformed raw data. While this study was considered exploratory in nature and no formal hypothesis testing was prespecified, p-values of less than 0.10 are generally considered 'significant' in Phase 2 proof-of-concept studies.
SYN-010 effects on breath methane were evaluated in a within group analysis by comparing the methane area under the curve (AUC) on a lactulose breath test at Day 7 vs. baseline Day 1 or Day 28 vs. baseline Day 1. After 7 days reductions in breath methane levels were seen in the 42 mg dose group (p=0.02) but not the 21 mg dose group (p=0.64). In contrast, after 28 days both the 21 mg (p=0.03) and 42 mg (p=0.01) dose groups showed reductions in breath methane levels.
Preliminary analysis of secondary efficacy assessment data indicates that an improvement in the stool frequency response for the 21 mg dose group (p=0.02) was apparent, while the 42 mg dose group (p=0.54) was numerically better demonstrating a positive trend. Additionally, an improvement in weekly abdominal pain intensity for the 42 mg dose group (p=0.08) was seen, while the 21 mg dose group (p=0.26) was numerically better demonstrating a positive trend. The definitions of these outcomes are consistent with the U.S. Food and Drug Administration (FDA) IBS guidance. There were no serious adverse events observed.
Synthetic Biologics' Publication in Alimentary Pharmacology & Therapeutics
Synthetic Biologics' peer-reviewed article titled "Inhibition of Methanogenic Archaea by Statins as a Targeted Management Strategy for Constipation and Related Disorders", evaluating the therapeutic potential and mechanism of action of certain statins in inhibiting the production of methane was recently published in the journal Alimentary Pharmacology & Therapeutics. The article describes observational and experimental studies which show a strong correlation between the production of methane by the dominant methane-producing microorganism in the gut, M. smithii, and slowed stool transit in the intestines commonly seen in patients with IBS-C. To access the online publication, please use the following link: http://onlinelibrary.wiley.com/doi/10.1111/apt.13469/epdf.
About Irritable Bowel Syndrome
IBS affects an estimated 10 to 15 percent of the population, or as many as 45 million people in North America. The illness affects both men and women; however, two-thirds of diagnosed sufferers are women. It has been reported that up to 20 percent of all IBS patients have IBS-C and current FDA-approved therapies for the treatment of IBS-C, which include prescription and over-the-counter laxatives, do little to treat the underlying cause of the disease. These products provide patients with temporary relief from the symptoms of constipation by elevating the amount of water which passes through the gastrointestinal tract, but tend to cause an IBS-C patient to swing from suffering from constipation, to suffering from diarrhea.
About Synthetic Biologics' Microbiome Clinical Program Seminar Webcast
Synthetic Biologics is hosting its Microbiome Clinical Program Seminar on Thursday, December 10, 2015 in New York City at 9:00 a.m. EST. The Seminar will provide clinical updates on Synthetic Biologics' gut microbiome candidates SYN-010 (treatment of IBS-C) and SYN-004 (prevention of C. difficile). A live webcast of the presentations will begin on December 10, 2015 at 9:00 a.m. EST and are scheduled to conclude by 11:30 a.m. EST. The live webcast of the event will be available via the internet at: http://bit.ly/1QWBpYN. The archived webcast will be available at the same URL, http://bit.ly/1QWBpYN, for at least 90 days following the Seminar.
About Synthetic Biologics, Inc.
Synthetic Biologics, Inc. (NYSE MKT: SYN) is a clinical stage company developing therapeutics to protect the microbiome while targeting pathogen-specific diseases. The Company's lead candidates in Phase 2 development include: (1) SYN-004 which is designed to protect the gut microbiome from the effects of certain commonly used intravenous (IV) antibiotics for the prevention of C. difficile infection and antibiotic-associated diarrhea (AAD), and (2) SYN-010 which is intended to reduce the impact of methane producing organisms in the gut microbiome to treat an underlying cause of irritable bowel syndrome with constipation (IBS-C). In addition, the Company is developing a Phase 2 oral estriol drug for the treatment of relapsing-remitting multiple sclerosis (MS) and cognitive dysfunction in MS, and in collaboration with Intrexon Corporation (NYSE: XON), a preclinical stage monoclonal antibody for the prevention and treatment of Pertussis and discovery stage biotherapeutics for the treatment of phenylketonuria (PKU). For more information, please visit Synthetic Biologics' website at www.syntheticbiologics.com.
This release includes forward-looking statements on Synthetic Biologics' current expectations and projections about future events. In some cases, forward-looking statements can be identified by terminology such as "may," "should," "potential," "continue," "expects," "anticipates," "intends," "plans," "believes," "estimates," and similar expressions. These statements are based upon current beliefs, expectations and assumptions and are subject to a number of risks and uncertainties, many of which are difficult to predict and include statements regarding the ability of SYN-010 to reduce methane production for the treatment of IBS-C while minimizing disruption to the microbiome, SYN-010's ability to act primarily in the intestinal lumen, the anticipated timing of the reporting of topline data from the second Phase 2 eight week extension study, timing of initiation of SYN-010 Phase 3 clinical trial, the belief that methane is a causative factor in constipation, and the potential market for SYN-010. The forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially from those set forth or implied by any forward-looking statements. Important factors that could cause actual results to differ materially from those reflected in Synthetic Biologics' forward-looking statements include, among others, a failure to receive the necessary regulatory approvals for commercialization of Synthetic Biologics' therapeutics, a failure of Synthetic Biologics' clinical trials, and those conducted by investigators, to be commenced or completed on time or to achieve desired results or results that are consistent to prior trial results, a failure of Synthetic Biologics' products for the prevention and treatment of diseases to be successfully developed or commercialized, Synthetic Biologics' inability to maintain its licensing agreements, or a failure by Synthetic Biologics or its strategic partners to successfully commercialize products and other factors described in Synthetic Biologics' report on Form 10-K for the year ended December 31, 2014 and any other filings with the SEC. The information in this release is provided only as of the date of this release, and Synthetic Biologics undertakes no obligation to update any forward-looking statements contained in this release on account of new information, future events, or otherwise, except as required by law.

Photo - http://photos.prnewswire.com/prnh/20151207/293881
Logo - http://photos.prnewswire.com/prnh/20130522/MM19465LOGO
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/synthetic-biologics-syn-010-lowered-breath-methane-and-improved-stool-frequency-in-a-phase-2-four-week-study-in-patients-with-irritable-bowel-syndrome-with-constipation-ibs-c-300189407.html
SOURCE Synthetic Biologics, Inc.





