Omniscient Neurotechnology today announced that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for Quicktome™, a digital brain mapping platform that allows neurosurgeons to visualize and understand a patient’s brain networks prior to performing life-changing brain surgery.
- World’s first and only brain connectomics planning software with regulatory clearance in United States, Canada and Australia
- Quicktome is a neurosurgical planning software which incorporates connectomics, the field of understanding brain connectivity into routine neurosurgical planning
SYDNEY, Australia--(BUSINESS WIRE)-- Omniscient Neurotechnology (“o8t™”), a pioneering brain mapping software company, today announced that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for Quicktome™, a digital brain mapping platform that allows neurosurgeons to visualize and understand a patient's brain networks prior to performing life-changing brain surgery. This announcement occurs on Glioblastoma Awareness Day, a day dedicated to increasing public understanding of the most common, complex, treatment-resistant, and deadliest type of brain cancer.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210721005355/en/
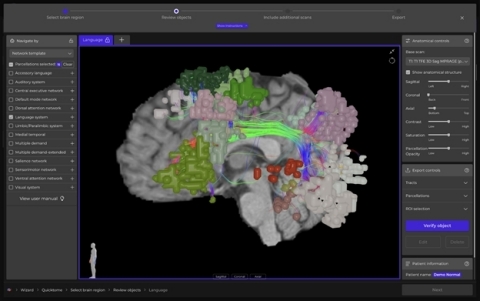
Quicktome allows neurosurgeons to visualize important brain networks, such as the language network, prior to surgery. (Photo: Business Wire)
In addition to FDA clearance in the United States, Quicktome has also received Health Canada approval as well as regulatory clearance by the Therapeutic Goods Administration (TGA) of Australia.
Quicktome is a neurosurgical planning software which incorporates “connectomics”, the field of understanding brain connectivity, into routine neurosurgical planning. Critically, by visualizing networks that are responsible for complex functions such as language, movement, and cognition, Quicktome assists neurosurgeons in making more informed decisions and reduces surgical uncertainty.
Utilizing cloud computing for large-volume data processing, and intuitive browser-based interfaces, Quicktome aims to streamline the process for neurosurgeons. The digital platform furthermore enables multidisciplinary collaboration in hospitals, and greater insight on a patient before and during surgery.
“We are thrilled to receive regulatory clearance for Quicktome. This is the first digital mapping platform designed to incorporate brain connectome data for neurosurgeons to improve patient outcomes,” said Stephen Scheeler, CEO, Omniscient Neurotechnology. “Until now, the tools that neurosurgeons have relied on have been no match for the brain’s complexity. Quicktome breaks information down into actionable insights to inform the impact each incision will have on the patient. Since its authorization, physicians are already relying on this technology to guide surgical planning. This is a significant milestone for Omniscient and, more importantly, begins a new era for neurosurgery for patients and physicians.”
“I wish I had technology like this when I started practicing,” said Dr. Michael Sughrue, Chief Medical Officer, Omniscient Neurotechnology, a neurosurgeon who has completed over 3,000 brain tumor removals. “When I learnt about connectomics, I realized the biggest impact I could make to the field of neurosurgery was to make Quicktome a routine source of insight for all neurosurgeons. With this ground-breaking technology, our hope is for a better quality of life for patients and families after brain surgery."
“Omniscient is the first to harness the power of brain connectomics for use in neurosurgery,” said Stephane Doyen, Chief Data Officer at Omniscient Neurotechnology. “Big data is changing everything we know about the brain. Modern neuroscience has shown that we are our brain networks, which control everything from movement to speech. Understanding how the brain is connected and what these connections mean will drastically help us better deliver healthcare, starting with neurosurgery.”
About Omniscient Neurotechnology
Omniscient Neurotechnology (o8t™) is a pioneering brain mapping company that is revolutionizing brain care with data. Using innovations in medical imaging and machine learning, Omniscient builds applications capable of mapping and analyzing “brain networks” which are formed by the electrical connections within a person’s brain. Such insights are illuminating the neural processes that make us human and helping clinicians and researchers understand, diagnose, and treat complex neurological and mental illnesses.
Omniscient is a leader in the field of “connectomics” – the study of the brain’s connections. It recently launched Quicktome™, the world’s first medical device to harness this vital data for neurological care, providing neurosurgeons brain network information prior to life-changing surgery. The company is headquartered in Sydney, Australia with offices globally. For more information, please visit www.o8t.com.
About Quicktome™
Quicktome™ is a digital brain mapping platform that allows neurosurgeons to visualize and understand a patient's brain networks prior to performing life-changing brain surgery. These brain networks are responsible for everything from language to movement to thought, and brain maps help inform surgical decision-making to preserve and protect them.
Quicktome™ analyzes millions of data points derived from a patient’s MRI. Designed by neurosurgeons and data scientists, the majority of the analysis takes place in the cloud and can be accessed easily on a desktop computer, allowing neurosurgeons and other healthcare professionals to focus on surgery.
The launch of Quicktome™ is a milestone that combines decades of connectomics research - the study of brain connections - with cutting-edge algorithms and cloud computing. Quicktome™ recently received regulatory clearance in the United States, Canada, and Australia.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210721005355/en/
Source: Omniscient Neurotechnology