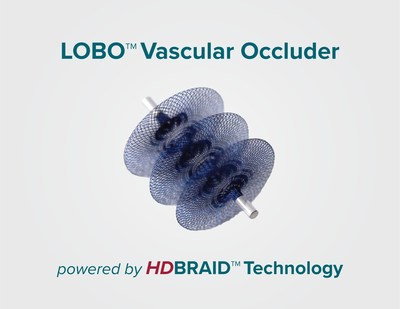
Okami Medical Inc., a medical device company, today announced the expansion of its LOBO™ Vascular Occlusion System product line with the U.S. FDA 510(k) clearance of the LOBO-5 Vascular Occluder.
|
ALISO VIEJO, Calif., Dec. 16, 2020 /PRNewswire/ -- Okami Medical Inc., a medical device company, today announced the expansion of its LOBO™ Vascular Occlusion System product line with the U.S. FDA 510(k) clearance of the LOBO-5 Vascular Occluder. The LOBO (LOw-profile Braided Occluder) system is uniquely designed to provide interventional physicians with a single-device, "one-and-done" solution for the occlusion of a wide range of peripheral arterial targets. The LOBO system combines neurovascular-derived HDBRAID™ technology with a patented design to create a highly occlusive structure for fast and efficient closure of blood vessels throughout the body. LOBO-5, the second offering in the company's product portfolio, is intended for use in 3 to 5 mm diameter vessels. LOBO-3, the company's first occluder, is intended for use in 1.5 to 3 mm vessels. The LOBO system is demonstrated in a newly released animation on the Okami Medical website (www.okamimedical.com). "The FDA clearance of LOBO-5 marks another milestone in our mission to provide patients and physicians with access to advanced technologies that address the numerous unmet clinical needs in peripheral vascular occlusion," said Bob Rosenbluth, PhD, President and CEO of Okami Medical. "LOBO-3 and LOBO-5, to be followed by LOBO-7 and LOBO-9, are designed and built to enable fast, predictable and complete occlusion of a diverse set of vascular targets without the need for multiple embolic devices." About Okami Medical Okami Medical, Inc. is a privately-held medical device company with a mission to address the evolving needs of patients and physicians through the development of innovative, versatile, and intuitive devices for the occlusion of peripheral vessels. The company was created in 2017 by Inceptus Medical, a medical device incubator. Okami is backed by members of the board of directors, U.S. Venture Partners (www.usvp.com) and medical device industry veterans. For more information, please visit www.okamimedical.com.
SOURCE Okami Medical Inc. |