-RSV F Vaccine was safe and well-tolerated
-Significant anti-F, PCA and microneutralizing antibody responses elicited in mothers
-Efficient antibody transfer from mothers to infants observed
-Results suggest potential to protect infants when they are most at risk
GAITHERSBURG, Md., Sept. 29, 2015 /PRNewswire/ -- Novavax, Inc., (Nasdaq: NVAX) a clinical-stage vaccine company focused on the discovery, development and commercialization of recombinant nanoparticle vaccines and adjuvants, today announced positive top-line data from a Phase 2 clinical trial of its RSV F-protein recombinant nanoparticle vaccine candidate (RSV F Vaccine) to protect infants via maternal immunization. Novavax also announced today that it has been awarded a grant of up to $89 million by the Bill & Melinda Gates Foundation to support development of the RSV F Vaccine Phase 3 clinical trial in pregnant women, planned to initiate during the first quarter of 2016.
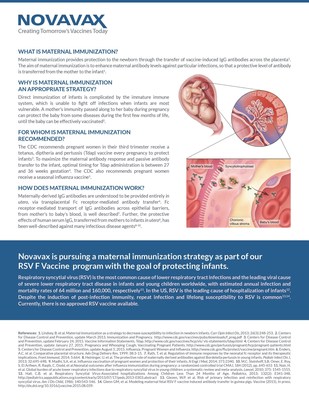
The purpose of the Phase 2 trial was to evaluate the safety and immunogenicity of the RSV F Vaccine in healthy pregnant women. The trial also assessed the transplacental transfer of maternal antibodies induced by the vaccine, the impact of maternal immunization on infant safety during the first year of life and RSV-specific antibody levels through the infants' first six months of life.
The trial was a randomized, blinded, placebo-controlled Phase 2 trial which enrolled 50 healthy pregnant women in their third trimester. Women were randomized to receive either placebo or 120 micrograms of RSV F Vaccine adjuvanted with 0.4 mg of aluminum phosphate. They were followed through the remainder of their pregnancies, delivery and for an additional 180 days postpartum to assess safety and immunogenicity as measured by serum RSV anti-F IgG, microneutralizing, and palivizumab-competing antibody (PCA) titers. Upon delivery, umbilical cord blood samples were obtained to determine the titers of RSV PCA, anti-F IgG, and microneutralizing titers in their infants. Additional serum samples from the infant participants were obtained over the following six month period to provide a preliminary estimate of the half-life of vaccine-induced maternal antibodies.
Women in the vaccinated group demonstrated a geometric mean 14-fold rise in anti-F IgG, 29-fold rise in PCA, and 2-fold rise in microneutralization titers. In contrast, women who received placebo demonstrated no significant change in their antibody levels. At delivery, the geometric mean anti-F IgG antibody titer in immunized women was 7,244 Elisa Units (EU) and on average, the infants' antibody titer equaled 100% of the mothers' anti-F IgG antibody titer. The geometric mean PCA antibody titer was 212 micrograms/mL in immunized mothers and the average PCA antibody transfer within the mother-infant pairs was 90%. Finally, geometric mean microneutralizing antibody titers in immunized mothers were 759 and 481 micrograms/mL for RSV/A and RSV/B respectively, and the average microneutralizing antibody transfer within the mother-infant pairs was 90% for RSV/A and 100% for RSV/B. The estimated half-lives of infant PCA, anti-F IgG, RSV/A and RSV/B microneutralizing antibodies, based on data through day 60, were 41, 30, 36 and 34 days, respectively.
"Maternal immunization is an elegant and accepted approach to protect infants in the first few months of life, the time period when they are most vulnerable to RSV," said Gregory Glenn, M.D., Senior Vice President, Research and Development. "These data are consistent with immune responses observed in our earlier trials of women of child-bearing age. As anticipated, infants received a significant bolus of all anti-RSV antibodies via transplacental transfer. Half of all hospitalizations due to RSV occur within the first three months of life and, based on these data, the RSV F Vaccine demonstrates the potential to protect infants when they are most at risk of infection."
"These data illustrate the promise of our RSV F Vaccine to protect infants via maternal immunization, demonstrating robust immune responses in women with antibody transfer to infants. We look forward to advancing our maternal immunization program to a Phase 3 clinical trial with the support of the Bill & Melinda Gates Foundation," said Stanley C. Erck, President and CEO. "We have now delivered positive top-line data from multiple clinical trials this quarter, including two from our RSV F Vaccine program. These announcements are not only significant achievements for our company, but important contributions to the field. We look forward to sustaining this momentum in the coming months, as we intend to initiate two pivotal Phase 3 trials."
A fact sheet on maternal immunization is available at the Novavax website, http://novavax.com/download/files/pipeline/151_Novavax_FactSheet_FIN_D_9x10.pdf
About RSV
Respiratory syncytial virus (RSV) is the most common cause of lower respiratory tract infections and the leading viral cause of severe lower respiratory tract disease in infants and young children worldwide, with estimated annual infection and mortality rates of 64 million and 160,000, respectively1. In the US, RSV is the leading cause of hospitalization of infants2. Despite the induction of post-infection immunity, repeat infection and lifelong susceptibility to RSV is common3,4. Currently, there is no approved RSV vaccine available. Palivizumab is a monoclonal antibody, licensed and sold by MedImmune as Synagis®, that targets the RSV F protein and is used for prophylaxis against RSV disease in high risk infants.
About Novavax
Novavax, Inc. (Nasdaq:NVAX) is a clinical-stage vaccine company committed to delivering novel products to prevent a broad range of infectious diseases. Our recombinant nanoparticles and Matrix-M adjuvant technology are the foundation for groundbreaking innovation that improves global health through safe and effective vaccines. Additional information about Novavax is available on the company's website, novavax.com.
References:
- Nair, H. et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet, 2010; 375: 1545-1555.
- Hall, C.B. et al. Respiratory Syncytial Virus-Associated hospitalizations Among Children Less Than 24 Months of Age. Pediatrics, 2013; 132(2): E341-348.
- Glezen, W.P. et al. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child, 1986; 140:543-546.
- Glenn GM, et al. Modeling maternal fetal RSV F vaccine induced antibody transfer in guinea pigs. Vaccine, 2015; In press. http://dx.doi.org/10.1016/j.vaccine.2015.08.039.
Contact: | Novavax, Inc. |
Barclay A. Phillips | |
SVP, Chief Financial Officer and Treasurer | |
Andrea N. Flynn, Ph.D. | |
Senior Manager, Investor Relations | |
240-268-2000 | |
Russo Partners, LLC | |
David Schull | |
Todd Davenport, Ph.D. | |
212-845-4271 |
Photo - http://photos.prnewswire.com/prnh/20150925/271009
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/novavax-announces-positive-top-line-data-from-phase-2-clinical-trial-of-rsv-f-vaccine-to-protect-infants-via-maternal-immunization-300149808.html
SOURCE Novavax, Inc.
 Help employers find you! Check out all the jobs and post your resume.
Help employers find you! Check out all the jobs and post your resume.




