With one month to go until World Brain Tumour Day on June 8, Kazia Therapeutics Limited, an Australian oncology-focused biotechnology company, is pleased to announce new safety data from its ongoing phase IIa study of GDC-0084 in newly-diagnosed patients with glioblastoma.
SYDNEY, May 8, 2019 /PRNewswire/ -- With one month to go until World Brain Tumour Day on June 8, Kazia Therapeutics Limited (ASX: KZA; NASDAQ: KZIA), an Australian oncology-focused biotechnology company, is pleased to announce new safety data from its ongoing phase IIa study of GDC-0084 in newly-diagnosed patients with glioblastoma (GBM).
Experience the interactive Multichannel News Release here: https://www.multivu.com/players/English/8542751-kazia-therapeutics-glioblastoma/
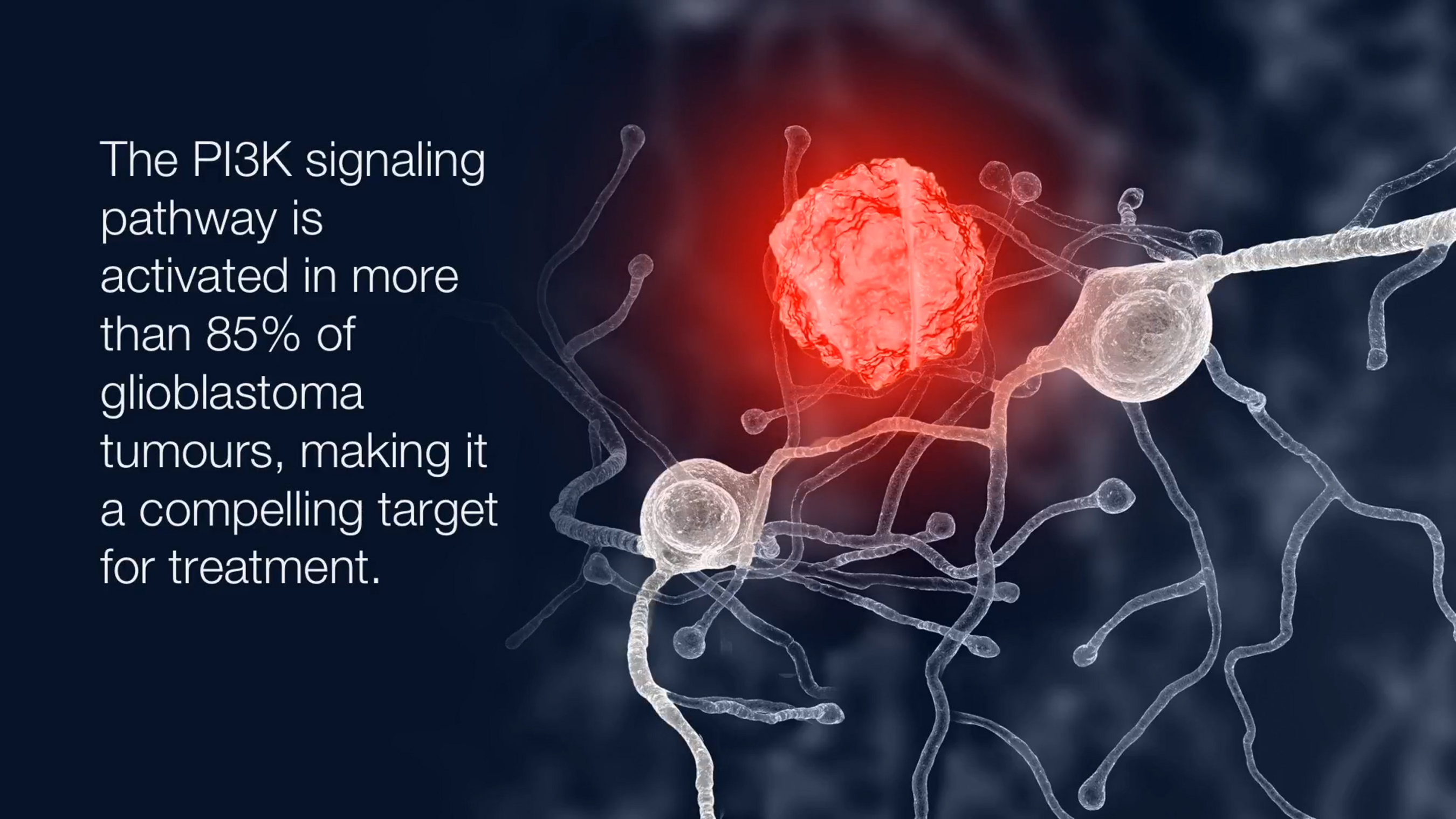
Licensed from Genentech in October 2016, GDC-0084 is a novel targeted therapy that inhibits the PI3K pathway, which is important in many forms of cancer and is activated in 85-90% of GBM cases. GDC-0084 is administered orally, once daily.
The phase II a study has determined a maximum tolerated dose (MTD) of 60mg, which is substantially higher than the 45mg dose found during Genentech's phase I study in patients with recurrent disease. Dose-limiting toxicities in this phase II a study included oral mucositis (mouth ulcers) and hyperglycemia (elevated blood sugar), both of which are expected effects of the PI3K inhibitor class of drugs.
Kazia's phase II a study of GDC-0084 is designed in two parts. The first part, which began dosing in September 2018 and is now complete, is a dose optimisation component, designed to determine if newly-diagnosed patients could tolerate a higher dose of GDC-0084 than advanced recurrent patients.
This part of the trial was expected to enroll between 6 and 18 patients, depending on the safety signal observed.
Ultimately, a total of eight patients were required for this part. The study will now immediately transition into the dose expansion cohort, in which an additional20 patients will be recruited. It is planned that these patients will receive a dose of 60mg. This second part of the study will be designed to provide confirmatory efficacy signals and is expected to report initial data in the fourth quarter of calendar 2019.
Dr James Garner, Chief Executive Officer of Kazia Therapeutics commented: "This is an important milestone and we are extremely pleased with the outcome. With GDC-0084, the side effects that we have seen to date have all been very typical for this type of drug: such as mouth ulcers and high blood sugar levels.
Critically, we have not seen any of the more serious side effects that have sometimes been observed with other drugs in the class, such as infections, liver toxicity, or gastrointestinal effects, which is very reassuring as we prepare to take the drug forward into the next phase of development. We believe that GDC-0084 has the potential to become an important new treatment option for patients with this very challenging disease."
Media contact: Gabriella Hold, gabriella.hold@irdepartment.com.au, +61 411 364 382
For more information on Kazia Therapeutics or GDC-0084, please visit: www.kaziatherapeutics.com


![]() View original content:http://www.prnewswire.com/news-releases/new-positive-data-from-phase-iia-trial-in-brain-cancer-patients-300846920.html
View original content:http://www.prnewswire.com/news-releases/new-positive-data-from-phase-iia-trial-in-brain-cancer-patients-300846920.html
SOURCE Kazia Therapeutics
Company Codes: Australia:KZA, NASDAQ-SMALL:KZIA




