Chengdu Kanghong Pharmaceutical Group, has received Special Protocol Assessments from the Food and Drug Administration for three new indications for its biologic drug conbercept.
CHENGDU, China, Feb. 3, 2021 /PRNewswire/ -- Chengdu Kanghong Pharmaceutical Group ("Kanghong", SHE: 002773), has received Special Protocol Assessments from the Food and Drug Administration for three new indications for its biologic drug conbercept. The drug is an anti-VEGF fusion protein, approved and marketed in China as Lumitin®, for the treatment of retinal diseases.
The additional indications are DME (Diabetic Macular Edema) and RVO (Central Retinal Vein Occlusion and Branch Retinal Vein Occlusion), which can cause blurred vision and even sudden vision loss. These new inclusions will potentially help over 21 million patients globally who suffered from DME, and approximately 28.06 million patients worldwide suffered from RVO. They may also facilitate a wider treatment range for conbercept in tackling age-related macular degeneration and blindness.
The President of Kanghong, Mr. Xiao Ke believes that "the purpose of our innovation and high standards is to improve the quality of patients' lives, and to develop other professional and groundbreaking pharmaceutical products in the future."
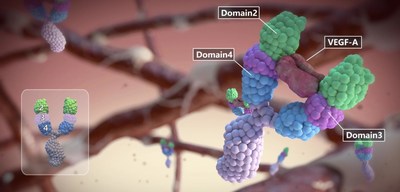
Conbercept is a hallmark product that showcases the company's ability to bring innovative drugs to patients who need them. Conbercept is a new generation anti-VEGF, a 100 percent humanized fusion protein that targets VEGF-B, placental growth factor (PlGF), and various isoforms of VEGF-A in the treatment of neovascular age-related macular degeneration ("nAMD"), choroidal neovascularization ("pmCNV"), and diabetic macular edema ("DME"). Conbercept's safety and effectiveness have been verified, as the drug has already been administered over one million injections.
Approved by the FDA and European Medicines Agency, conbercept is moved to Phase III of clinical trials and undertaken in over 300 research institutions across 30 countries. The independent PANDA trials completed 36-week primary endpoint visits of enrolled patients in October 2020. The main clinical endpoint data expected in early 2021, the drug is to be FDA-approved by 2022. Conbercept is expected to launch globally in 2023.
Centers for Disease and Control Prevention have found that vision problems are primarily age-related caused by AMD, cataract, diabetic retinopathy, and glaucoma. Kanghong has been able to provide sustainable solutions for all of these conditions. One such example is CLASS®, a minimally invasive and innovative laser-assisted surgical treatment program for the long-term treatment of glaucoma. The technology originated from the Israeli company IOPtima, acquired by Kanghong in 2017, of paramount importance for the internationalization of Kanghong.
Kanghong remains dedicated to innovation, quality, and cooperation. The company has made clinical demand the focal point of its development strategy and keeps investing in developing innovative drugs.
Since its inception, Kanghong has dedicated to innovation to address clinical needs. Kanghong's development pipeline includes three projects developing antibodies to treat retinal diseases: KH621, KH615, and KH634, a bi-specific antibody.
About Chengdu Kanghong Pharmaceutical Group
Chengdu Kanghong Pharmaceutical Group is dedicated to improving ophthalmic care through developing and commercializing its innovative products for a wide range of ocular conditions. Founded in 1996, the Group became a publicly listed company in China in 2015.
For more information about Kanghong Pharmaceutical, please visit: https://en.cnkh.com/
Media Contact: media@cnkh.com, 028-87509966
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/more-patients-set-to-benefit-as-kanghong-pharmaceutical-targets-new-indications-with-conbercept-301221126.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/more-patients-set-to-benefit-as-kanghong-pharmaceutical-targets-new-indications-with-conbercept-301221126.html
SOURCE Chengdu Kanghong Pharmaceutical Group
Company Codes: Shenzhen:002773