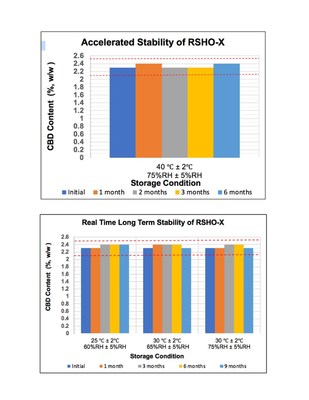
The stability study was conducted in strict compliance with FDA/ICH guidelines (Q1A-R2).
|
SAN DIEGO, June 12, 2019 /PRNewswire/ -- Medical Marijuana, Inc. (OTC: MJNA) (the "Company"), the first-ever publicly traded cannabis company in the United States that launched the world's first-ever cannabis-derived nutraceutical products, brands and supply chain, announced today that the Company has reached a major milestone for long-term stability testing on its flagship THC-free cannabidiol (CBD) oil product Real Scientific Hemp Oil-X™ (RSHO-XTM). The stability study was conducted in strict compliance with FDA/ICH guidelines (Q1A-R2).
"We're excited to offer consumers and retail partners the transparency and security of knowing that our products are reliable and stable throughout several climatic conditions and through transportation," said Medical Marijuana, Inc. CEO Dr. Stuart Titus. "Our company set the standards for many CBD testing protocols in the U.S. and intends to continue doing so with this study. Our customers strongly rely on the quality, potency and safety of RSHO-X, which has been tested in two successful pilot clinical studies. Therefore, we take it seriously to keep the quality standard criteria higher for our products and provide accurate and specific safety and expiration information." "The results from the study provide significant evidence that RSHO-X has a shelf-life of at least 12 months at different and extreme climate conditions. This helps the company ensure to its customers and retailers that the integrity of the product will not be compromised while in transportation or while being stored for future use," said Medical Marijuana, Inc. Vice President of Project Analysis and R&D, Dr. Levan Darjania. "The purpose of this accelerated and long-term testing was to evaluate the stability profile of RSHO-X and to assess the product's performance and safety as well as the acceptability according to the FDA-ICH Guideline on stability testing." The data obtained from this study also reveals indications about the behavior of RSHO-X™ over determined time intervals while facing the different environmental conditions to which it may be exposed from the production date until the end of its validity. The study was outsourced to one of the most qualified cGLP/cGMP compliant Contract Research Organizations (CROs) with attained ISO 17025 accreditation and certification. During the course of the stability study, multiple samples of RSHO-X™ were exposed to various climatic zones associated environmental conditions covering the U.S., Europe, Mexico and Brazil. CBD quantitation tests were performed using fully validated, proprietary analytical UPLC-PDA and LC-MS/MS methods reflecting the specificity and selectivity toward RSHO-X™, including data elements and parameters such as accuracy, precision, repeatability, ruggedness, system suitability, linearity, specificity, dilution integrity and dynamic range. In addition to CBD monitoring and quantitation, the stability samples were tested for appearance, odor, specific gravity, viscosity, package compatibility and complete microbial test characteristics. The test results showed that no change (more than 5%) in the CBD content and other specifications were observed and the container closure system was faultless at both long-term and accelerated test conditions. The positive 6-month accelerated and 9-month long-term stability test results suggest that RSHO-X™ is a stable formulation. A preliminary shelf-life of 12 months was derived from the primary and supportive data. The stability testing will be continued up to 24 months. A corresponding shelf-life of 24 months is anticipated. To learn more about the study or Medical Marijuana, Inc., please visit: www.medicalmarijuanainc.com. About Medical Marijuana, Inc. To see Medical Marijuana, Inc.'s corporate video, click here. FORWARD-LOOKING DISCLAIMER FOOD AND DRUG ADMINISTRATION (FDA) DISCLOSURE LEGAL DISCLOSURE CONTACT: Investor Relations Contact:
SOURCE Medical Marijuana, Inc. |
||
Company Codes: OTC-PINK:MJNA, OTC-QB:MJNA |